Abstract
Purpose
The predictive value of 18F-FDG PET in patients with relapsing/refractory lymphoma who are receiving high-dose chemotherapy and autologous stem cell transplantation (ASCT) remains a matter of debate. Seminal reports on pretransplant ASCT indicated an adverse prognosis in patients with positive FDG PET scans. The lack of a uniform outcome measure along with the mixed histologies in various studies have hampered efforts to quantify this prognostic value.
Methods
A MEDLINE review of published trials up to April 2009 identified 16 studies involving pretransplant FDG PET scans in lymphoma. Where progression-free survival (PFS) and overall survival (OS) were set as the main outcome measures, time-to-event data analysis was used to calculate the overall prognostic value of a pretransplant FDG-PET scan.
Results
Pooled survival data from seven eligible studies suggested a worse PFS in patients with a positive FDG PET study (HR 3.23, 95% CI 2.14 to 4.87). The OS pooled from six eligible studies was also significantly worse among patients with a positive FDG PET study (HR 4.53, 95% CI 2.50 to 8.22). No statistically significant heterogeneity was observed between studies for either outcome.
Conclusion
Despite the documented clinical heterogeneity between studies, meta-analysis data confirmed the prognostic impact of pretransplant FDG PET in patients with lymphoma and provided a uniform measure of the association for both progression and survival after ASCT.
Similar content being viewed by others
Introduction
The role of 18F-FDG PET scanning in the posttreatment evaluation of malignant lymphomas has been the subject of intense research in recent years. Its diagnostic performance, as compared with conventional imaging methods, has been systematically reviewed [1–5]. The value of 18F-FDG PET in patients with lymphoma has mainly been assessed in the spheres of pretreatment staging, early evaluation during treatment and posttreatment evaluation. Its value in other domains, such as follow-up after remission or postinduction restaging, remains to be assessed. In the seminal studies of Becherer et al. [6] and Cremerius et al. [7], positive pretransplant 18F-FDG PET studies were associated with a poor outcome in patients receiving salvage therapy and autologous stem cell transplantation (ASCT). Patients with a positive study showed a higher relapse rate, as well as decreased overall survival (OS). Numerous studies have since identified pretransplant PET scans as being highly prognostic with regard to OS and progression-free survival (PFS) after ASCT. Many have included a wide range of histologies, including Hodgkin’s lymphoma (Hodgkin’s disease, HD) and non-Hodgkin’s lymphoma (NHL). In studies involving patients with mixed histologies, PFS at 2 years was as much as 82% better among those with a negative pre-ASCT PET scan. In studies involving only patients with NHL, failure-free survival was as high as 43% better among those with a negative pre-ASCT PET scan [8]. However, any attempt to clarify the association between the pretransplant PET scan and long-term outcome is partially hampered by inclusion of various histologies, small sample sizes and the lack of a common outcome measure between different studies.
We conducted a comprehensive review of relevant published trials and a meta-analysis of survival data to determine the value of a positive pretransplant PET scan in predicting PFS and OS in patients undergoing ASCT for malignant lymphoma.
Methods
A systematic search of the MEDLINE database up to April 2009 was initially conducted, using ‘positron emission tomography’, ‘transplantation’ and ‘lymphoma’ as search terms. All searches were limited to studies in humans. Reviews and other editorial material were excluded. Inclusion criteria were a priori defined to identify studies that (a) included patients with lymphoma receiving ASCT who had received a pretransplant evaluation including a PET scan and (b) reported OS and/or PFS as the main outcome measures. We therefore excluded studies not reporting survival data and studies that combined results from other types of functional imaging (such as gallium studies). On the contrary, any prior treatments and different histologies were not considered as exclusion criteria.
The full text of all potentially relevant articles was retrieved and the references were reviewed manually to identify any additional studies. Finally, all relevant publications were reviewed independently by two authors (P.D.Z., L.S.P.) and the following information was sought from each article: author, year of publication, type of study design, sample size, histology and effect measures of survival (OS, PFS) stratified into PET-positive vs. PET-negative arms. Any disagreement was resolved by consensus between the two authors.
Survival data from each study were analysed in terms of Kaplan-Meier curves, unless hazard ratios (HRs) were reported, and compared to calculate HRs and 95% confidence intervals (CIs) as previously described by Parmar et al. [9] and Tierney et al. [10]. In brief, effects were measured from the observed minus expected difference (O−E) and variance (V) generated using the reported summary statistics, by the one-step approximation exp[(O−E)/V]. These effects were combined to estimate the overall (pooled) effect of the PET-positive vs. PET-negative arm. An HR <1 denotes survival benefit from a positive PET scan whereas an HR >1 indicates an increased risk of progression and death. Statistical heterogeneity was measured using the chi-squared Q test (p < 0.10 was considered to represent significant statistical heterogeneity) and the I2 statistic, as described by Higgins et al. [11].
Results
The initial search retrieved 86 articles. A total of 43 studies were immediately excluded, including 19 case reports, 18 reviews and 6 nonhuman studies. The full text of the remaining 43 studies was retrieved and examined on the basis of relevance, according to the predefined criteria. Of these 43 , 27 were eliminated as they did not report the use of pretransplant PET in lymphoma. Thus, 16 potentially relevant publications remained eligible for further analysis [6, 7, 12–25]. Table 1 presents the summary of the 16 studies on pretransplant PET in lymphoma, with regard to main demographic, clinical and outcome measures. Most studies addressed the combined value of the FDG PET scan in predicting survival outcomes without discriminating HD from NHL. Increasing use of hybrid PET/CT scanners [18–20, 22, 23] replacing dedicated PET scanners in the earlier studies was apparent.
All studies with available data confirmed the high value of the pretransplant PET scan in predicting both progression and survival outcomes. Results of ASCT are unidirectional and in favour of PET responders as being less likely to advance and more likely to experience long-term survival [6, 7, 12–16, 18, 19, 22–25] (Table 1). In the only study in patients with highly aggressive lymphoma who had been allotransplanted, this favourable prognostic value is also preserved [21].
Four studies with nonextractable PFS/OS data [13, 15, 17, 20], one study on allogeneic transplant [21] and two studies that presented combined data from PET studies and 67Ga scans [18, 23] were not included in the meta-analysis. A manual cross-reference of eligible studies and review articles added no further studies. A total of nine studies were used for meta-analysis data extraction [6, 7, 12, 14, 16, 19, 22, 24, 25]. Two studies did not report PFS data to be extracted [6, 19] but did report OS data. One study [14] provided PFS data without OS data. Two studies presented OS information [6, 12], but the absence of failures in Kaplan-Maier curves produced HR estimates reaching infinity according to the methodology used, and therefore could not be used to extract OS data. Finally, there were seven included studies with usable information on PFS and six with usable information on OS. The selection procedure is presented in Fig. 1 using in the form of a QUOROM flow diagram [26].
Cremerius et al. [7] investigated the predictive value of PET before and after front-line high-dose therapy/ASCT in 22 patients with NHL. Six of seven patients without at least a partial metabolic response had relapsed, whereas 10/15 patients with complete or partial metabolic response remained in complete remission. However, the criterion to define response (>25% decrease in standard uptake volume) is controversial as it is mainly used for solid tumours in the neoadjuvant setting rather than in lymphoma patients. Spaepen et al. [12] demonstrated that the pretransplant PET scan is of high prognostic value in patients with aggressive lymphomas still chemosensitive to salvage chemotherapy. Although a negative scan cannot exclude minimal residual disease leading to late relapses, it was of favourable prognostic value as only 3/30 patients had relapsed. On the other hand, patients with residual uptake before transplantation had a worse outcome as 26/30 eventually relapsed after ASCT and 16 patients died. In the study by Schot et al. [14], 46 NHL/HD patients in chemosensitive relapse were evaluated and 65% of PET-positive patients showed progressive disease, whereas 33% of PET-negative patients progressed, with a median follow up of 20 months. In the same study, a reduction in tumour volume of >90% (compared to the PET scan before salvage therapy) was not associated with a significant difference in PFS, whereas a reduction in intensity of >90% was associated with a significantly better PFS (25% at 2 years for <90% vs 62% for >90% intensity reduction).
In the study by Svoboda et al. [16] in relapsed or primary refractory NHL/HD, median PFS was 19 months for the PET-negative group vs. only 5 months for the PET-positive group. The median OS was not reached in the PET-negative group and was 19 months in the PET-positive group. Additionally, for the patients who had both chemoresistant disease by CT and a positive PET scan, the HR for progression was 4.2 when compared to the favourable group of those with chemosensitive disease both by CT and negative PET scan. The study by Fillmont et al. [19] introduced the use of hybrid PET/CT scanners in pretransplant evaluation and confirmed the prognostic impact of a positive study on OS (53% vs. 92% for negative patients at 1 year). Importantly, a subset analysis indicated that the impact of PET did not differ either between various histological subtypes or between the timing of ASCT (i.e. as first-line treatment for both refractory disease and recurrent disease). In the study by Crocchiolo et al. [22] in NHL/HD patients, the 5-year OS was significantly better (90%) in those with a negative pretransplant study than in those with a positive study (55%).
Derenzini et al. [24] confirmed the favourable association between a negative pre-transplant PET study and all significant outcomes, including PFS and OS (87.2% vs 34.7% for positive PET and 93.7% vs. 66.6% for positive PET, respectively). Pretransplant PET was the single independent prognostic factor for both outcomes in multivariable analysis. It should be noted however that this cohort of patients only included patients with diffuse large B-cell lymphoma (DLBCL) and patients with grade 3 follicular lymphoma. The larger study in number of patients and duration of follow-up by Hoppe et al. [25] confirmed the results of the previous groups in patients with relapsed/refractory DLBCL. Finally, despite the differences in how the studies describe PET responses (using either visual assessment or SUV-based criteria), the results of an improved outcome are concordant when patients have a negative FDG PET scan prior to high-dose therapy and ASCT.
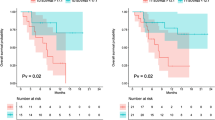
The combined analysis of time-to-event data for disease progression (PFS) in seven studies suggested that a positive pretransplant PET scan was associated with an increased risk of progression compared to a negative PET result (Fig. 2a). The pooled effect was significant under the fixed-effects model (HR 3.23, 95% CI 2.14 to 4.87) with no evidence of quantitative (statistical) heterogeneity between included studies (I2 2%). With regard to OS (Fig. 2b), reported data were also unidirectional to decreased survival for PET-positive patients. The pooled effect of the six analysed studies was also significant under the fixed-effects model (HR 4.53, 95% CI 2.50 to 8.22) with no evidence of statistical heterogeneity between studies.
a Forest plot of seven included studies (PFS). Pooled effect (HR) of a PET-positive scan on PFS. The data were drawn from 386 patients with lymphoma who had a pretransplant PET study. b Forest plot of six included studies. Pooled effect (HR) of a PET-positive scan on OS. The data were drawn from 340 patients with lymphoma who had a pretransplant PET study
Discussion
FDG PET scans following second-line chemotherapy, before ASCT in malignant lymphomas have significant prognostic value and have been incorporated into treatment algorithms. Our results confirm the findings of independent groups showing that patients who fail to achieve a negative PET scan before ASCT have a significantly worse outcome. We have also managed to summarize time-to-event data in a single predictive figure, despite the documented clinical heterogeneity between included studies. In clinical terms, we have demonstrated that at any given time after salvage treatment and ASCT, the risk of progression is three times higher while the risk of death is four times higher in patients who not achieving a pretransplant PET-negative scan. The method we used overcomes one of the major obstacles within the independent studies, that of the lack of a uniform duration of follow-up after ASCT. The calculation of a common HR bypasses the need to have similar follow-up times.
However, true clinical heterogeneity was still present, given that studies may report the value of pretransplant PET in B-cell lymphomas only [20, 23–25], HD only [18], or most commonly, may combine the value in various histological subtypes [6, 7, 12–17, 19, 21–25]. Each of the previous reports had a different design, size, entry criteria and methodology, which resulted in a wide range of values obtained. To our surprise, the magnitude of this qualitative heterogeneity was not reflected as statistical heterogeneity in the data analysis. There is increasing evidence that the FDG PET scan is a stronger predictive tool than other clinically validated scores. Spaepen et al. [12] suggested that pretransplant PET provides a stronger prognosis than the IPI score [27]. Schot et al. [17] developed a combined clinical risk score for both NHL and HD; this, along with FDG PET results, were independent predictors of outcome. Impressively, histology was not a significant predictive factor in the univariate analysis. Therefore, we may speculate that the lack of statistical heterogeneity provides indirect evidence that a positive pretransplant FDG PET scan has a prognostic impact in lymphoma patients undergoing ASCT after salvage treatment, which may attenuate prior differences in histology, clinical staging and first-line therapies.
However, it should be strongly emphasized that a positive PET scan before transplantation does not justify patient exclusion from this treatment option. Salvage chemotherapy and ASCT remain the best legitimate option for relapsed lymphoma with a significant curative potential and should not be abandoned on the strength of a positive functional study. The FDG PET scan can be used for risk stratification in terms of progression and survival, but a positive scan should not be used as an exclusion criterion.
References
Isasi CR, Lu P, Blaufox MD. A metaanalysis of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography in the staging and restaging of patients with lymphoma. Cancer 2005;104:1066–74.
Zijlstra JM, Lindauer-van der Werf G, Hoekstra OS, et al. 18F-fluoro-deoxyglucose positron emission tomography for post-treatment evaluation of malignant lymphoma: a systematic review. Haematologica 2006;91:522–9.
Terasawa T, Nihashi T, Hotta T, et al. 18F-FDG PET for posttherapy assessment of Hodgkin’s disease and aggressive non-Hodgkin’s lymphoma: a systematic review. J Nucl Med. 2008;49:13–21.
Poulou LS, Karianakis G, Ziakas PD. FDG PET scan strategies and long-term outcomes after first-line therapy in Hodgkin’s disease. Eur J Radiol. 2009;70:499–506.
Ziakas PD, Poulou LS. Improving outcome after positive interim PET in advanced Hodgkin’s disease: reality vs expectation. Eur J Nucl Med Mol Imaging. 2008;35:1573–5.
Becherer A, Mitterbauer M, Jaeger U, et al. Positron emission tomography with [18F]2-fluoro-D-2-deoxyglucose (FDG-PET) predicts relapse of malignant lymphoma after high-dose therapy with stem cell transplantation. Leukemia 2002;16:260–7.
Cremerius U, Fabry U, Wildberger JE, et al. Pre-transplant positron emission tomography (PET) using fluorine-18-fluoro-deoxyglucose (FDG) predicts outcome in patients treated with high-dose chemotherapy and autologous stem cell transplantation for non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2002;30:103–11.
Johnston PB, Wiseman GA, Micallef IN. Positron emission tomography using F-18 fluorodeoxyglucose pre- and post-autologous stem cell transplant in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2008;41:919–25.
Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34.
Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.
Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood 2003;102:53–9.
Filmont JE, Czernin J, Yap C, et al. Value of F-18 fluorodeoxyglucose positron emission tomography for predicting the clinical outcome of patients with aggressive lymphoma prior to and after autologous stem-cell transplantation. Chest 2003;124:608–13.
Schot B, van Imhoff G, Pruim J, et al. Predictive value of early 18F-fluoro-deoxyglucose positron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123:282–7.
Schot BW, Pruim J, van Imhoff GW, et al. The role of serial pre-transplantation positron emission tomography in predicting progressive disease in relapsed lymphoma. Haematologica 2006;91:490–5.
Svoboda J, Andreadis C, Elstrom R, et al. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2006;38:211–6.
Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood 2007;109:486–91.
Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer 2007;109:2481–9.
Filmont JE, Gisselbrecht C, Cuenca X, et al. The impact of pre- and post-transplantation positron emission tomography using 18-fluorodeoxyglucose on poor-prognosis lymphoma patients undergoing autologous stem cell transplantation. Cancer 2007;110:1361–9.
Bishu S, Quigley JM, Schmitz J, et al. F-18-fluoro-deoxy-glucose positron emission tomography in the assessment of peripheral T-cell lymphomas. Leuk Lymphoma 2007;48:1531–8.
Yoshimi A, Izutsu K, Takahashi M, et al. Conventional allogeneic hematopoietic stem cell transplantation for lymphoma may overcome the poor prognosis associated with a positive FDG-PET scan before transplantation. Am J Hematol. 2008;83:477–81.
Crocchiolo R, Canevari C, Assanelli A, et al. Pre-transplant 18FDG-PET predicts outcome in lymphoma patients treated with high-dose sequential chemotherapy followed by autologous stem cell transplantation. Leuk Lymphoma 2008;49:727–33.
Alousi AM, Saliba RM, Okoroji GJ, et al. Disease staging with positron emission tomography or gallium scanning and use of rituximab predict outcome for patients with diffuse large B-cell lymphoma treated with autologous stem cell transplantation. Br J Haematol. 2008;142:786–92.
Derenzini E, Musuraca G, Fanti S, et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer 2008;113:2496–503.
Hoppe BS, Moskowitz CH, Zhang Z, et al. The role of FDG-PET imaging and involved field radiotherapy in relapsed or refractory diffuse large B-cell lymphoma. Bone Marrow Transplant 2009;43:941–8.
Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlledtrials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354(9193):1896–900.
The International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–94.
Financial support
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poulou, L.S., Thanos, L. & Ziakas, P.D. Unifying the predictive value of pretransplant FDG PET in patients with lymphoma: a review and meta-analysis of published trials. Eur J Nucl Med Mol Imaging 37, 156–162 (2010). https://doi.org/10.1007/s00259-009-1258-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1258-y