Abstract
Purpose
(R,S)-N-(4-Bromo-2-fluorophenyl)-6-methoxy-7-((1-methyl-3-piperidinyl)methoxy)-4-quinazolinamine (PAQ) is a tyrosine kinase inhibitor with high affinity for the vascular endothelial growth factor receptor 2 (VEGFR-2), which plays an important role in tumour angiogenesis. The aim of this work was to develop and evaluate in mice the 11C-labelled analogue as an in vivo tracer for VEGFR-2 expression in solid tumours.
Methods
[11C]PAQ was synthesized by an N-methylation of desmethyl-PAQ using [11C]methyl iodide. The tracer’s pharmacokinetic properties and its distribution in both subcutaneous and intraperitoneal tumour models were evaluated with positron emission tomography (PET). [18F]FDG was used as a reference tracer for tumour growth. PET results were corroborated by ex vivo and in vitro phosphor imaging and immunohistochemical analyses.
Results
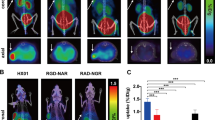
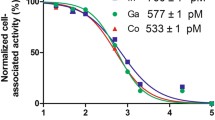
In vitro assays and PET in healthy animals revealed low tracer metabolism, limited excretion over 60 min and a saturable and irreversible binding. Radiotracer uptake in subcutaneous tumour masses was low, while focal areas of high uptake (up to 8% ID/g) were observed in regions connecting the tumour to the host. Uptake was similarly high but more distributed in tumours growing within the peritoneum. The pattern of radiotracer uptake was generally different from that of the metabolic tracer [18F]FDG and correlated well with variations in VEGFR-2 expression determined ex vivo by immunohistochemical analysis.
Conclusion
These results suggest that [11C]PAQ has potential as a noninvasive PET tracer for in vivo imaging of VEGFR-2 expression in angiogenic “hot spots”.









Similar content being viewed by others
References
Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86:353–64. doi: 10.1016/S0092-8674(00)80108-7.
Bouck N, Stellmach V, Hsu S. How tumors become angiogenic. Adv Cancer Res 1996;69:135–74. doi: 10.1016/S0065-230X(08)60862-3.
Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249–57. doi: 10.1038/35025220.
Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971;285:1182–6.
Folkman J. Angiogenesis: an organizing principle for drug discovery. Nat Rev Drug Discov 2007;6:273–86. doi: 10.1038/nrd2115.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989;246(4935):1306–9. doi: 10.1126/science.2479986.
Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev 1997;18:4–25. doi: 10.1210/er.18.1.4.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76. doi: 10.1038/nm0603-669.
Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 2002;20(21):4368–80. doi: 10.1200/JCO.2002.10.088.
Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci U S A 1995;92:768–72. doi: 10.1073/pnas.92.3.768.
Veeravagu A, Hsu AR, Cai W, Hou LC, Tse VC, Chen X. Vascular endothelial growth factor and vascular endothelial growth factor receptor inhibitors as anti-angiogenic agents in cancer therapy. Recent Patents Anticancer Drug Discov 2007;2:59–71.
Longo R, Gasparini G. Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol 2007;60:151–70. doi: 10.1007/s00280-006-0403-6.
Stephen RM, Gilles RJ. Promise and progress for functional and molecular imaging of response to targeted therapies. Pharm Res 2007;24:1172–85. doi: 10.1007/s11095-007-9250-3.
Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med 2008;49:113S–28S. doi: 10.2967/jnumed.107.045922.
Cai W, Chen X. Multimodality imaging of vascular endothelial growth factor and vascular endothelial growth factor receptor expression. Front Biosci 2007;12:4267–79. doi: 10.2741/2386.
Collingridge DR, Carroll VA, Glaser M, Aboagye EO, Osman S, Hutchinson OC, et al. The development of [124I]iodinated-VG76e: a novel tracer for imaging vascular endothelial growth factor in vivo using positron emission tomography. Cancer Res 2002;62:5912–9.
Jayson GC, Zweit J, Jackson A, Mulatero C, Julyan P, Ranson M, Broughton L, et al. Molecular imaging and biological evaluation of HuMV833 anti-VEGF antibody: implications for trial design of antiangiogenic antibodies. J Natl Cancer Inst 2002;94:1484–93.
Nagengast WB, de Vries EG, Hospers GA, Mulder NH, de Jong JR, Hollema H, et al. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J Nucl Med 2007;48:1313–9. doi: 10.2967/jnumed.107.041301.
Cai W, Chen K, Mohamedali KA, Cao Q, Gambhir SS, Rosenblum MG, et al. PET of vascular endothelial growth factor receptor expression. J Nucl Med 2006;47:2048–56.
Backer MV, Levashova Z, Patel V, Jehning BT, Claffey K, Blankenberg FG, et al. Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med 2007;13:504–9. doi: 10.1038/nm1522.
Rodriguez-Porcel M, Cai W, Gheysens O, Willmann JK, Chen K, Wang H. Imaging of VEGF receptor in a rat myocardial infarction model using PET. J Nucl Med 2008;49:667–73. doi: 10.2967/jnumed.107.040576.
Willmann JK, Chen K, Wang H, Paulmurugan R, Rollins M, Cai W, et al. Monitoring of the biological response to murine hindlimb ischemia with 64Cu-labeled vascular endothelial growth factor-121 positron emission tomography. Circulation 2008;117:915–22. doi: 10.1161/CIRCULATIONAHA.107.733220.
Wang H, Cai W, Chen K, Li ZB, Kashefi A, He L, et al. A new PET tracer specific for vascular endothelial growth factor receptor 2. Eur J Nucl Med Mol Imaging 2007;34:2001–10. doi: 10.1007/s00259-007-0524-0.
Ciardiello F, Bianco R, Caputo R, Caputo R, Damiano V, Troiani T, et al. Antitumor activity of ZD6474, a vascular endothelial growth factor receptor tyrosine kinase inhibitor, in human cancer cells with acquired resistance to antiepidermal growth factor receptor therapy. Clin Cancer Res 2004;10:784–93. doi: 10.1158/1078-0432.CCR-1100-03.
Herbst RS, Heymach JV, O’Reilly MS, Onn A, Ryan AJ. Vandetanib (ZD6474): an orally available receptor tyrosine kinase inhibitor that selectively targets pathways critical for tumor growth and angiogenesis. Expert Opin Investig Drugs 2007;16:239–49. doi: 10.1517/13543784.16.2.239.
Hennequin LF, Stokes ES, Thomas AP, Johnstone C, Plé PA, Ogilvie DJ, et al. Novel 4-anilinoquinazolines with C-7 basic side chains: design and structure activity relationship of a series of potent, orally active, VEGF receptor tyrosine kinase inhibitors. J Med Chem 2002;45:1300–12. doi: 10.1021/jm011022e.
Rivera J, Jayasuriya N, Rane D, Keertikar K, Ferreira JA, Chao J. Synthesis of substituted 1H-imidazol-1-ylmethylpiperidines. Facile separation of 1,4- and 1,5-disubstituted imidazoles. Tetrahedron Lett 2002;43:8917–9. doi: 10.1016/S0040-4039(02)02157-3.
Thorell JO, Samén E, Stone-Elander S. Synthesis and in vitro metabolism studies of the VEGFR2 inhibitor (R,S) N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-[11C]methyl-3-piperidinyl)methoxy]-4-quinazolinamine. J Labelled Comp Radiopharm 2007;50:S323. doi:10.1002/jlcr.1242.
Lee SY, Choe YS, Kim DH, Park BN, Kim SE, Choi Y, et al. A simple and efficient in vitro method for metabolism studies of radiotracers. Nucl Med Biol 2001;28:391–5. doi: 10.1016/S0969-8051(01)00203-7.
Rovero S, Amici A, Carlo ED, Bei R, Nanni P, Quaglino E, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol 2000;165:5133–42.
Levchenko T, Veitonmäki N, Lundkvist A, Gerhardt H, Ming Y, Berggren K, et al. Therapeutic antibodies targeting angiomotin inhibit angiogenesis in vivo. FASEB J 2008;22:880–9. doi: 10.1096/fj.07-9509com.
Cao Y, O’Reilly MS, Marshall B, Flynn E, Ji RW, Folkman J. Expression of angiostatin cDNA in a murine fibrosarcoma suppresses primary tumor growth and produces long-term dormancy of metastases. J Clin Invest 1998;101:1055–63. doi: 10.1172/JCI1558.
Gustafson DL, Bradshaw-Pierce EL, Merz AL, Zirrolli JA. Tissue distribution and metabolism of the tyrosine kinase inhibitor ZD6474 (Zactima) in tumor-bearing nude mice following oral dosing. J Pharmacol Exp Ther 2006;318:872–80. doi: 10.1124/jpet.106.102376.
Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol 2006;168:639–48. doi: 10.2353/ajpath.2006.050834.
Mi Y, Lou L. ZD6474 reverses multidrug resistance by directly inhibiting the function of P-glycoprotein. Br J Cancer 2007;97:934–40. doi: 10.1038/sj.bjc.6603985.
Ozvegy-Laczka C, Hegedus T, Varady G, Ujhelly O, Schuetz JD, Varadi A, et al. High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol 2004;65:1485–95. doi: 10.1124/mol.65.6.1485.
Miller KD, Sweeney CJ, Sledge GW. Redefining the target: chemotherapeutics as antiangiogenics. J Clin Oncol 2001;19:1195–206.
Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs 1999;17:343–59. doi: 10.1023/A:1006326203858.
Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev 1999;17:279–84. doi: 10.1023/A:1006140513233.
Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell 1998;94:715–25. doi: 10.1016/S0092-8674(00)81731-6.
Norrby K. In vivo models of angiogenesis. J Cell Mol Med 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x.
Nagy JA, Meyers MS, Masse EM, Herzberg KT, Dvorak HF. Pathogenesis of ascites tumor growth: fibrinogen influx and fibrin accumulation in tissues lining the peritoneal cavity. Cancer Res 1995;55:369–75.
Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, et al. Pancreatic islet production of vascular endothelial growth factor-a is essential for islet vascularization, revascularization, and function. Diabetes 2006;55:2974–85. doi: 10.2337/db06-0690.
Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 2008;68:4340–6. doi: 10.1158/0008-5472.CAN-07-6705.
Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 2000;60:1388–93.
Siemeister G, Martiny-Baron G, Marmé D. The pivotal role of VEGF in tumor angiogenesis: molecular facts and therapeutic opportunities. Cancer Metastasis Rev 1998;17:241–8. doi: 10.1023/A:1006027124696.
Chen K, Cai W, Li Z-B, Wang H, Chen X. Quantitative PET imaging of VEGF receptor expression. Mol Imaging Biol 2009;11:15–22.
Acknowledgments
This project was financially support by the Karolinska Institutet, Swedish Cancer Society, Cancerföreningen Stockholm, Swedish Research Council, EC FP6 (LSHC-CT-2004-505785) and EUCAAD FP7, which is gratefully acknowledged. The authors also thank Apoteket’s Central Laboratory (Apoteket, Stockholm, Sweden) for performing the LC-MS analyses, Klas Wiman for loan of the phosphor imaging device and Karolinska Institutet’s Mouse Tissue Unit for the IHC analyses. This article does not necessarily reflect the views of the EC. The EC is not liable for any use that may be made of the information contained herein.
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samén, E., Thorell, JO., Lu, L. et al. Synthesis and preclinical evaluation of [11C]PAQ as a PET imaging tracer for VEGFR-2. Eur J Nucl Med Mol Imaging 36, 1283–1295 (2009). https://doi.org/10.1007/s00259-009-1111-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-009-1111-3