Abstract
Purpose
11C-PK11195 is a radiopharmaceutical for in vivo assessment of peripheral benzodiazepine receptor (PBR) activity using PET. We sought to clarify the metabolic fate of 11C-PK11195 in a test–retest setting using radio-HPLC in comparison with radio-TLC, and the whole-body distribution in humans.
Materials and methods
In order to evaluate the reproducibility of radio-HPLC metabolite analyses, ten patients with Alzheimer’s disease (AD) underwent two successive 11C-PK11195 examinations on separate days. For comparison of different analytical methods, plasma samples from seven patients were also analysed by radio-TLC. In addition, we evaluated the whole-body distribution of 11C-PK11195 and its uptake in the brain.
Results
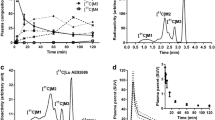
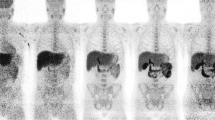
The level of unmetabolized 11C-PK11195 decreased slowly from 96.3 ± 1.6% (mean±SD) at 5 min to 62.7 ± 8.3% at 40 min after injection. Large individual variation was observed in the amount of plasma 11C-PK11195 radiometabolites. The whole-body distribution of 11C-PK11195 showed the highest radioactivity levels in urinary bladder, adrenal gland, liver, salivary glands, heart, kidneys, and vertebral column. In addition, the hip bone and breast bone were clearly visualized by PET. In patients with AD, 11C-PK11195 uptake in the brain was the highest in the basal ganglia and thalamus, followed by the cortical grey matter regions and the cerebellum. Low 11C-PK11195 uptake was observed in the white matter.
Conclusion
Our results indicate that 11C-PK11195 is eliminated both through the renal and hepatobiliary systems. Careful analysis of plasma metabolites is required to determine the accurate arterial input function for quantitative PET measurement.







Similar content being viewed by others
References
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, et al. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 2006;27:402–409.
Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int 2002;40:475–486.
Krueger KE. Molecular and functional properties of mitochondrial benzodiazepine receptors. Biochim Biophys Acta 1995;241:453–470.
Skrabanek L, Campagne F. TissueInfo: high-throughput identification of tissue expression profiles and specificity. Nucleic Acids Res 2001;29:E102.
Huminiecki L, Lloyd AT, Wolfe KH. Congruence of tissue expression profiles from Gene Expression Atlas, SAGEmap and TissueInfo databases. BMC Genomics 2003;4:31.
Junck L, Olson JM, Ciliax BJ, Koeppe RA, Watkins GL, Jewett DM, et al. PET imaging of human gliomas with ligands for the peripheral benzodiazepine binding site. Ann Neurol 1989;26:752–758.
Vowinckel E, Reutens D, Becher B, Verge G, Evans A, Owens T, et al. PK11195 binding to the peripheral benzodiazepine receptor as a marker of microglia activation in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci Res 1997;50:345–353.
Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain 2000;123:2321–2337.
Turner MR, Cagnin A, Turkheimer FE, Miller CC, Shaw CE, Brooks DJ, et al. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis 2004;15:601–609.
Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol 2005;57:168–175.
Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, et al. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology 2000;55:1052–1054.
Kropholler MA, Boellaard R, Schuitemaker A, van Berckel BN, Luurtsema G, Windhorst AD, et al. Development of a tracer kinetic plasma input model for (R)-[11C]PK11195 brain studies. J Cereb Blood Flow Metab 2005;25:842–851.
De Vos F, Dumont F, Santens P, Slegers G, Dierckx R, De Reuck J. High-performance liquid chromatographic determination of [11C]-1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide in mouse plasma and tissue and in human plasma. J Chromatogr B Biomed Sci Appl 1999;736:61–66.
Charbonneau P, Syrota A, Crouzel C, Valois J-M, Prenant C, Crouzel M. Peripheral-type benzodiazepine receptors in the living heart characterized by positron emission tomography. Circulation 1986;73:476–483.
Pike VW, Halldin C, Crouzel C, Barré L, Nutt DJ, Osman S, et al. Radioligands for PET studies of central benzodiazepine receptors and PK (peripheral benzodiazepine) binding sites – current status. Nucl Med Biol 1993;20:503–525.
Luthra SK, Osman S, Turton DR, Vaja V, Dowsett K, Brady F. An automated system based on solid phase extraction and HPLC for the routine determination in plasma of unchanged [11C]-L-deprenyl; [11C]diprenorphine; [11C]flumazenil; [11C]raclopride; and [11C]Scherring 23390. J Labelled Compd Radiopharm 1993;32:518–520.
Debruyne JC, Versijpt J, Van Laere KJ, De Vos F, Keppens J, Strijckmans K, et al. PET visualization of microglia in multiple sclerosis patients using [11C]PK11195. Eur J Neurol 2003;10:257–264.
DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner. J Nucl Med 1994;35:1398–1406.
Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab 2007;27:369–377.
Greuter HNJM, van Ophemert PLB, Luurtsema G, van Berckel BN, Franssen EJ, Windhorst BD, et al. Optimizing an online SPE–HPLC method for analysis of (R)-[11C]1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide [(R)-[11C]PK11195] and its metabolites in humans. Nucl Med Biol 2005;32:307–312.
Wala EP, Sloan JW, Jing X. Pharmacokinetics of the peripheral benzodiazepine receptor antagonist, PK 11195, in rats. The effect of dose and gender. Pharmacol Res 2000;41:461–468.
Ferry A, Jaillon P, Lecocq B, Lecocq V, Jozefczak C. Pharmacokinetics and effects on exercise heart rate of PK 11195 (52028 RP), an antagonist of peripheral benzodiazepine receptors, in healthy volunteers. Fundam Clin Pharmacol 1989;3:383–392.
Cleij MC, Airbirhio FJ, Baron JC, Clark JC. Base-promoted dechlorination of (R)-[11C]PK 11195. J Labelled Compd Radiopharm 2003;46:S88.
Diorio D, Weiner SA, Butterworth RF, Meaney Mi, Suranyi-Cadotte BE. Peripheral benzodiazepine binding sites in Alzheimer’s disease frontal and temporal cortex. Neurobiol Aging 1991;12:255–258.
Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, et al. In-vivo measurement of activated microglia in dementia. Lancet 2001;358:461–467.
Virsu P, Laitinen I, Pöyhönen T, Någren K, Roivainen A. In vivo biodistribution, biokinetics and blood metabolism of 11C-PK11195 in rats – a PET tracer for peripheral benzodiazepine receptor. Eur J Nucl Med Mol Imaging 2005;32:P752.
Acknowledgments
The authors thank the staff of the Turku PET Centre for performing PET imaging. Henri Sipilä is thanked for technical assistance in the clinical laboratory measurements. This study was financially supported by Turku University Hospital (EVO 13856) and Turku Imanet, GE Healthcare Medical Diagnostics, Turku, Finland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roivainen, A., Någren, K., Hirvonen, J. et al. Whole-body distribution and metabolism of [N-methyl-11C](R)-1-(2-chlorophenyl)-N-(1-methylpropyl)-3-isoquinolinecarboxamide in humans; an imaging agent for in vivo assessment of peripheral benzodiazepine receptor activity with positron emission tomography. Eur J Nucl Med Mol Imaging 36, 671–682 (2009). https://doi.org/10.1007/s00259-008-1000-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-1000-1