Abstract
Introduction
Positron emission tomography (PET) is the gold standard for non-invasive assessment of myocardial viability and allows accurate detection of coronary artery disease by assessment of myocardial perfusion. Magnetic resonance imaging (MRI) provides high resolution anatomical images that allow accurate evaluation of ventricular structure and function together with detection of myocardial infarction.
Objective
Potential hybrid PET/MR tomography may potentially facilitate the combination of information from these imaging modalities in cardiology. Furthermore, the combination of anatomical MRI images with the high sensitivity of PET for detecting molecular targets may extent the application of these modalities to the characterization of atherosclerotic plaques and to the evaluation of angiogenetic or stem cell therapies, for example.
Discussion
This article reviews studies using MRI and PET in parallel to compare their performance in cardiac applications together with the potential benefits and applications provided by hybrid PET/MRI systems.
Similar content being viewed by others
Introduction
Positron emission tomography (PET) has contributed significantly to the advances in our understanding of cardiac physiology and pathophysiology for more than 20 years. In addition to being a powerful research tool in cardiology, recent technical development and improved availability facilitated also its routine clinical use in cardiology. PET is the most reliable non-invasive tool for the identification of myocardial viability and also allows accurate assessment of myocardial perfusion and coronary artery disease (CAD), which is known to be the leading cause of mortality in adults. Assessment of myocardial perfusion plays an important role in the diagnostic work-up of patients with suspected CAD as well as in the assessment of prognosis and guiding of therapy in patients with established CAD [1, 2]. Imaging of myocardial viability with PET has been shown to identify heart failure patients who are at increased risk of death, which can be effectively reduced by surgical treatment [3]. Heart failure is also a common condition affecting approximately 2% of the general adult population in Europe with rapidly increasing prevalence with age pointing to the large need for diagnostic studies [4].
A few years ago hybrid imaging systems with multi-slice computed tomography (MSCT) and PET were introduced and are now considered the imaging modalities-of-choice for a variety of clinical indications [5]. Although their use is primarily driven by oncological applications, interest in cardiac studies is increasing. Hybrid tomographs make it possible to perform a rapid and comprehensive evaluation of functional and anatomical severity of CAD by assessment of myocardial perfusion and non-invasive angiography. On the one hand, optimal evaluation of haemodynamic consequences requires integration of anatomical information with demonstration of myocardial ischaemia. On the other hand, myocardial infarction and sudden cardiac death result from the rupture of plaques that do not necessitate significant flow limitations [6]. Several features, such as severe luminal obstruction, thin fibrous cap, large lipid core, and the presence of active inflammation are characteristic of vulnerable plaques. Non-invasive imaging of plaque burden in combination with the features of plaque vulnerability might, therefore, lead to an improved assessment of individuals at risk of acute coronary events and help to refine estimates of the cardiovascular risk in the subclinical phase of atherosclerosis [6].
Magnetic resonance imaging (MRI) has rapidly developed into a versatile tool for investigating cardiovascular diseases. While the current interest in cardiac hybrid PET/CT technology is mainly based on the creation of attenuation correction maps, detection of atherosclerotic plaque burden and non-invasive angiography, MRI has additional well-established cardiac applications to offer, including evaluation of cardiac structure, assessment of ventricular function, and detection of myocardial infarction. The combination of this technique without ionizing radiation with PET is attractive in many applications as summarized in Table 1. In addition to contributing to the existing applications, combining MRI with its ability to produce high-resolution anatomical images and PET with its high sensitivity for the detection of molecular targets may help to extend these modalities into new applications, such as atherosclerotic plaque characterization, imaging of stem cells and evaluation of angiogenesis.
This review summarizes the experience from comparative studies using sequential parallel PET and MRI in different cardiac applications. Technical difficulties together with potential benefits of combining information from these techniques are discussed in present as well as emerging clinical cardiac applications.
Co-registration and fusion of cardiac MRI and PET
To perform a combined analysis of sequential MRI and PET studies, images must be aligned accurately in the spatial domain. However, a particular challenge in cardiac studies is that the three spatial dimensions must be supplemented with a time dimension in order to correct for continuous motion during respiratory and cardiac cycles. Cardiac gating is routine in MRI and also possible in PET. Moreover, the integration of respiratory triggering is achieved using “navigators” (MRI) or list-mode acquisitions (PET) [7]. However, variable acquisition times between PET and MRI cause additional problems for exact timing of events. MRI scans are typically performed within few seconds during breath-holds while PET scans have a minimum duration of 5 min. However, spatial co-registration is rarely addressed in studies using separate, sequential MRI and PET scans. In most cases, mapping of the regional properties, such as wall motion or contrast medium uptake, is performed according to a simplified, 17 segment model suggested by the American Heart Association [8]. Thus, it is fair to assume that the synergistic information is not fully exploited.
Misalignment is mostly caused by significant patient motion, respiration, and cardiac contraction. The first two are very challenging, as they are involuntary and can produce irregular patterns. In their review, Mäkela et al. summarized the co-registration accuracy for intra- and intermodal cardiac applications [9]. For alignment of repeated MRI acquisitions accuracies of 1.5–3.0 mm were reported. Co-registration accuracy of repeated PET studies was on the order of 1.0–2.5 mm. Finally, alignment of gated PET with gated MRI images showed an accuracy of 2.0±1.6 mm [10]. In another study a misalignment of 2.8±0.5 mm was calculated using thorax and lung surfaces [11].
From a practical point of view, the alignment issue of MRI and PET images is similar to the co-registration of CT and PET or SPECT studies. In this case, correct alignment of nuclear data and CT is a prerequisite for successful correction of photon attenuation and thus has received a lot of attention [12, 13]. Both studies lead to the conclusion that co-registration of any data not acquired simultaneously is a major problem in inter- and intra-modal examinations.
Integrated PET/MR tomographs
In the absence of a prototype suitable for cardiac use, we can speculate on the use of combinded PET/MR. However, a few relevant items should be briefly discussed:
-
A hybrid PET/MR system could potentially provide some other benefits in comparison to stand alone scanners or hybrid PET/CT as summarized for specific applications in Table 1.
-
In a hybrid system, real-time MR-based motion detection and correction could be applied since cardiac and respiratory gating can be image-based without external sensors. This could allow partial-volume correction and event-based correction of patient motion during the PET acquisition.
-
The lack of exposure to ionizing radiation and iodinated contrast agents makes hybrid PET/MRI attractive when compared with PET/CT. Although radiation exposure from CT when used for attenuation correction can be limited [14], the radiation dose from coronary CT angiography (CTA) is >10 mSv in recent clinical trials has been >10 mSv, which is clearly higher than from standard radiography or X-ray angiography [15]. Moreover, precise alignment between emission and “transmission” PET images may benefit from repeated, simultaneously acquired attenuation maps.
-
From a practical point of view, advances in CT technology are likely to result in substantial changes in hardware in hybrid PET/CT systems while advances in MRI are frequently based on imaging sequences and less complex pieces of hardware, such as coils, which makes upgrading easier and less expensive.
-
Both cardiac MRI and PET examinations can be rather time consuming. Thus, improvements in patient compliance as a result of reduced scan time could be significant, particularly in patients with dyspnoea due to heart failure who have difficulty holding their breath during MRI acquisitions.
-
Increased patient throughout based on reduced scan times is also likely to be cost-effective, but the absolute hardware costs remain to be seen. In Fig. 1, a hypothetical hybrid protocol for an extensive cardiac PET/MRI study is shown in comparison to a corresponding MRI protocol. Nonetheless, similar integrated protocols appear demanding on both staff and imaging systems. Irrespectively, such an extended protocol will be not likely to become an everyday standard.
-
Of note, a hybrid imaging device will require interplay between different specialties, namely cardiology, radiology and nuclear medicine that remains a matter of discussion – both from a political perspective as well as regarding cross-training issues for the technical and medical personnel.
Diagnosis of coronary artery disease
Myocardial perfusion imaging
The available evidence indicates that myocardial perfusion PET provides the most accurate non-invasive means of diagnosing obstructive CAD with sensitivity and specificity of about 90% [1]. Recent evidence also indicates the usefulness of PET for determining prognosis [16, 17]. The unique feature of PET is that myocardial blood flow (MBF) and coronary flow reserve (CFR) can be quantified in absolute terms using tracer kinetic modelling. 13N-labelled ammonia (NH3) and 15O-labelled water have been well validated for this purpose in experimental studies using microspheres as well as in CAD patients [18, 19]. The potential of the generator-produced potassium analogue 82Rb still remains under investigation [20]. The quantification of MBF and CFR is of diagnostic importance particularly in those patients with extensive multivessel CAD [1, 21]. It also allows evaluation of very early changes in coronary vasoreactivity and the progression or regression of CAD [21].
Dynamic MR imaging of the first-pass signal changes in the myocardium after injection of a fast bolus of gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) allows detection of coronary artery stenoses [22]. A meta-analysis of single-centre studies including mainly patients with a high prevalence of disease indicated good sensitivity and specificity (91% and 81%, respectively) of stress perfusion MRI in the diagnosis of CAD [23]. Recently, a randomized, prospective multicentre trial involving 234 patients carried out in experienced centres demonstrated that diagnostic accuracy of myocardial perfusion MRI with pharmacological stress was comparable to that of SPECT (areas under the ROC curve 86% and 75%, respectively) for detection of significant coronary stenosis defined as >50% stenosis [24]. Only a few studies have directly correlated the MRI-derived flow with quantitative PET data from human subjects. One study compared 13NH3 PET and MRI, using a pixel-wise up-slope parameter of the signal intensity curve during contrast medium passage as a flow index. This study, performed on 18 healthy subjects and 48 CAD patients, revealed a good correlation between the number of pathological segments per patient and high accuracy when implementing PET as reference (sensitivity 91%, specificity 94%) [25]. However, a similar study showed that MRI significantly underestimated CFR. The MRI threshold for pathological CFR was very low as compared to that with PET (1.3 versus 2.5) [26]. Technical limitations of first-pass perfusion MRI include difficulty in the direct assessment of the arterial input function, low extraction of Gd-DTPA in the presence of high MBF with substantial “roll off” as is known in SPECT, difficulty in modelling the myocardial kinetics of Gd-DTPA due to its rapid diffusion into the extracellular space, and limited spatial coverage of the left ventricle (LV) (3–5 slices). Currently, more studies are needed to fully elucidate the potential of MRI perfusion imaging in detection of CAD in comparison to nuclear imaging techniques [27].
Coronary angiography and vessel wall imaging
Given the better spatial resolution (approximately 0.4 mm in CT vs. 0.8 mm in MRI) and, thus, better diagnostic accuracy, MSCT appears to be the preferred technique for non-invasive coronary angiography today. However, technical advances in non-invasive coronary angiography by MRI have been rapid and it has the potential to become a valuable diagnostic tool. A multicentre, single-vendor trial of 3-D coronary MR angiography in 109 patients showed sensitivity, specificity and negative predictive values of 93%, 58%, and 81% for detection of >50% diameter stenosis of the proximal and middle segments of coronary arteries using quantitative coronary angiography as the reference [28]. Notably, 16% of segments were of nondiagnostic quality. In addition to evaluating the patency of the vascular lumen, MRI is capable of directly detecting atherosclerotic plaques and identifying their structural components, although the use of intracoronary coils makes this approach more invasive [29]. Although the small dimensions of the coronary artery walls make their evaluation challenging, there are reports indicating the feasibility of visualization of an increased coronary artery wall thickness in patients with atherosclerosis using black-blood pulse sequences [30].
Molecular imaging may provide an opportunity to enhance contrast in plaque imaging by combining the high sensitivity of PET to demonstrate accumulation of a specific contrast agent with MRI characterization of the morphology of individual plaques. Macrophages are the target of many molecular imaging approaches in atherosclerosis, because inflammation is a key pathophysiological process throughout the progression of atherosclerosis [31]. Rudd et al. were the first to demonstrate in humans that 18F-fluorodeoxyglucose (FDG) is accumulated by metabolically active macrophages in the atherosclerotic carotid artery, which can be visualized using PET [32]. However, 18F-FDG is a relatively non-specific marker of metabolic activity and, thus, there is interest in developing more specific tracers to target specific macrophage receptors, protease activity, angiogenesis, and apoptosis, as reviewed elsewhere [33]. In addition to providing an essential anatomical reference for the atherosclerotic wall of small vessels, MRI could provide a means to perform partial volume corrections of the PET signal and, thus, facilitate its quantification
Assessment of heart failure
Ventricular function
Determination of LV function in gated PET studies is based on the use of partial volume effects. As the effective spatial resolution in clinical cardiac PET scans is approximately 6–10 mm FWHM (full-width at half-maximum), changes in wall thickness can be estimated in terms of changes in regional count rates during cardiac contraction. Using algorithm-dependent assumptions, such as homogeneous tracer distribution over the myocardial wall, geometrical models can be used to estimate endo- and epicardial borders. This approach has been extensively implemented and validated in SPECT imaging [34] and has also been used in comparative MRI and PET studies [35, 36]. However, while being technically feasible, the accuracy of the assessment of global and regional LV function by PET is limited by the low resolution of the PET compared to that of MRI. Here, MRI is clearly the modality-of-choice to quantitate cardiac function, such as volumes, mass, ejection fraction and regional wall thickening [37]. Determination of LV function is essential for the diagnosis of heart failure and its prognostic value is well established. Therefore, its combination with any other imaging parameter is likely to provide incremental information. Moreover, some measures used for evaluation of heart failure therapies, such as efficiency of cardiac work, combine PET acquisitions of myocardial oxidative metabolism by the clearance kinetics of 11C-acetate with the assessment of ventricular function that could be ideally done using hybrid PET/MRI tomography [38].
Myocardial viability
Ischaemic myocardium that is dysfunctional but viable has the potential for recovery of contractile function after revascularization. Evaluation of myocardial glucose utilization with 18F-FDG PET is considered as the most reliable tool to assess myocardial viability [39]. Its quantitative nature allows assessment of the amount of viable tissue as a continuum from fully viable, through partially viable in the areas of non-transmural infarction, to non-viable scar. Contrast-enhanced MRI appears to be a promising alternative capable of visualizing transmural distribution of viable and infarcted myocardium with excellent spatial resolution [40]. Contrast-enhanced MRI of myocardial infarction is based on the delayed-enhancement technique using inversion-recovery prepared T1-weighted gradient-echo pulse sequences after intravenous administration of Gd-DTPA. Infarcted myocardium appears enhanced relative to normal myocardium when imaged with a delay (typically 5–20 min) after intravenous injection of the contrast agent due to different wash-out kinetics [41, 42].
A comparison of delayed-enhancement MRI with 18F-FDG PET and 13NH3 in 31 patients with ischaemic heart failure revealed that the location and extent of infarct scarring as delineated by delayed enhancement correlated very well with the non-viable segments from PET [43] (Fig. 2). The main source of difference between the methods was related to the presence of non-transmural enhancement in regions that were viable on PET. This can be explained by the higher spatial resolution of MRI compared with nuclear imaging methods that makes delayed-enhancement suitable for the detection of small areas of subendocardial infarcts. An improved contractile performance after intervention is commonly considered as the gold standard for assessing myocardial viability, although benefits of revascularization do not appear to be limited to improved function. In an initial study by Kim et al. in 41 patients with chronic ischaemic heart disease, dysfunctional segments with less than 25% of delayed enhancement were likely to recover after complete revascularization [44]. In contrast, segments with more than 50% of delayed enhancement had a low probability (<10%) of functional improvement. Comparable positive predictive values (73%) for functional recovery 6 months after revascularization were found for the lack of delayed enhancement (cut-off value less than 50% transmural scar) and the presence of preserved 18F-FDG uptake (cut-off value more than 50% of normal myocardium) as assessed by PET as the reference method to detect viable vs. non-viable myocardium [45].
Co-visualization of a delayed-enhancement MR image with perfusion assessed by 13N-ammonia PET (left MRI, middle fusion MRI/PET, right PET). The subendocardial enhancement pattern in the MR image is matched with a reduced 13N-ammonia uptake because of partial volume effects as well as reduced MBF. Because of the limited spatial resolution of PET, however, these effects cannot be separated
A recent meta-analysis of diagnostic studies indicated that 18F-FDG PET has a higher sensitivity than delayed enhancement MRI (92% vs. 84%) in predicting functional recovery upon revascularization, while specificities were comparable (63%) [39]. The relatively low specificity of the techniques indicates that a substantial percentage of segments that are classified as viable by the imaging techniques do not improve after revascularization. Although there are limited data on their combined use, adjunctive PET and MRI markers of viability, such as reduced diastolic wall thickness, lack of contractile response to inotropic stimulation with a low dose of dobutamine, preserved epicardial rim of viable myocardium, and preserved perfusion have been proposed to help refine the classification of dysfunctional segments as clinically viable or non-viable [46, 47].
In addition to improved detection of viability, multimodal imaging approaches could contribute to the clarification of the complex pathophysiological basis underlying the LV dysfunction in ischaemic heart failure. An example is presented in Fig. 3 where the presence of transmural delayed enhancement is associated with normal 18F-FDG uptake after experimental ischaemia reperfusion possibly related to inflammation [48].
Co-registered 18F-FDG (a) and delayed enhancement MR image (b) in a rat model of 20 min ischaemia followed by 24 h of reperfusion. 18F-FDG was administrated under fasting conditions. Surprisingly, increased 18F-FDG uptake is seen (arrows) in the area of acute myocardial infarction, as seen on the delayed-enhancement MR image (arrows) (reproduced with permission from reference [48])
Non-invasive detection of viable myocardium in chronic LV dysfunction associated with CAD has important clinical implications for the treatment of patients. Although limited by the lack of large randomized clinical trials, a meta-analysis of retrospective data has indicated that such patients are also at substantial risk of death, which can be effectively reduced by successful revascularization [3]. Furthermore, pre-operative assessment of viability may identify patients who are at low risk of serious perioperative complications [49]. The prognostic significance of myocardial scars detected by delayed enhancement is currently under investigation [50]. Among 159 patients with clinical suspicion of CAD but without a history of myocardial infarction, the presence of even small amounts of delayed enhancement was associated with a high risk of major adverse clinical events (hazard ratio 8.29 for death, acute myocardial infarction or unstable angina pectoris, hospitalization for heart failure, and life-threatening arrhythmias requiring defibrillation) and cardiac death (hazard ratio 10.9) during a follow-up period of 16 months [50].
It is important to emphasize that delayed-enhancement MRI is specific for either acute myocardial infarction or scar of old infarction, but not for hibernating myocardium. Plain infarct size carries well-established prognostic value that is also expected to apply to MRI. However, in contrast, the prognostic value of PET relies on the relative comparison of flow and glucose uptake (mismatch), which has been shown experimentally and clinically to reflect viable, but jeopardized myocardium. Several studies have indicated that there is an association between PET “mismatch” and adverse clinical outcome [3]. There is a need for prospective clinical trials comparing measures of infarction and either hibernation or stunning obtained with MRI and PET to obtain prognostic data to better understand whether they provide complementary clinical value.
Sympathetic nervous system
The sympathetic nervous system plays an important role in regulating cardiac performance to meet changing circulatory demands by increasing heart rate and the force of cardiac contraction. Several PET tracers, such as the catecholamine analogue 11C-labelled meta-hydroxyephedrine (HED) and beta-adrenoceptor tracers can be used to visualize global and local defects in myocardial sympathetic innervation caused by various cardiac diseases such as CAD, heart failure and arrhythmogenic disorders [51]. A large number of single-centre studies have indicated that these defects are related to a worse outcome in patients with heart failure, and thus innervation imaging might provide prognostic information as well as guide selection of therapies [52]. The combination of PET and MRI might facilitate risk stratification by allowing immediate combination of innervation studies with LV function, which is currently the major prognostic indicator and criterion for therapy selection [4]. Moreover, it might allow focusing the innervation studies on specific myocardial areas of interest, such as the viable border zone of myocardial infarction, a potential substrate of ventricular tachycardia [53].
Angiogenesis
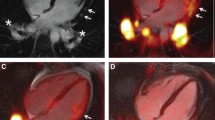
Formation of new capillaries from pre-existing vasculature, known as angiogenesis, occurs in response to ischaemia and inflammation being part of the healing process after ischaemic tissue injury. RGD peptides can be radiolabelled to produce the PET tracer 18F-galacto-RGD that allows non-invasive imaging of the expression of αvβ3 integrin, a cell membrane glycoprotein receptor that is highly expressed in the endothelium of angiogenic vessels [54]. Recent studies have provided evidence that 18F-galacto-RGD accumulates in chemically injured myocardium and the uptake can be visualized by PET imaging in the area of myocardial infarct scar [55]. Since expression of αvβ3 integrin reflects regional myocardial responses to injury, imaging of its expression might be useful for monitoring the effectiveness of various therapies aimed at improving the healing of myocardium after injury. Figure 4 demonstrates 18F-galacto-RGD imaging of integrin expression in response to myocardial infarction [56]. In this patient, the delayed-enhancement MRI signal allowed exact localization of the 18F-galacto-RGD uptake in the region of myocardial infarction.
Integration of delayed enhancement MRI, PET perfusion and avb3 integrin expression images in a patient who had had reperfused myocardial infarction 2 weeks previously. a, d Four chamber view (a) and two chamber view (b) show delayed enhancement (arrows) extending from the anterior wall to the apical region. b, e Fusion of 13N-ammonia PET shows severely reduced MBF in the region of delayed enhancement (arrows). c, f Focal 18F-RGD signal colocalized to the infarcted area as seen by delayed-enhancement MRI. g Polar map of MBF assessed by 13N-ammonia indicates severely reduced flow in the myocardial region perfused by the distal left anterior descending artery. h Co-registered 18F-RGD signal corresponding to the regions of severely reduced 13N-ammonia flow signal, reflecting the extent the of αvβ3 expression within the infarcted area (modified with permission from reference [56])
Cell and gene therapies
Stem cell transplantation is a promising therapeutic option for heart diseases related to cardiomyocyte death, in particular acute myocardial infarction and subsequent heart failure. Direct visualization of stem cells by imaging methods may provide novel insights into the number of cells surviving in the heart and help validate novel regenerative cell therapies non-invasively [57]. Reporter gene PET imaging has been shown to be feasible for visualization of the survival of stem cells in experimental models of myocardial infarction. An example of imaging of myocardial expression of the reporter gene used to assess delivery of adenoviral gene therapy after intramyocardial injection is shown in Fig. 5 [58]. Combined MR imaging allows localization of the reporter gene expression at high spatial resolution together with anatomical and functional characterization of the target myocardium.
a–c Co-registration and fusion display in PET “hot spot” imaging using 124I-2′-fluoro-2′-deoxy-5-iodo-1-β-d-arabinofuranosyluracil (124I-FIAU) as marker of gene expression in a pig model. Without anatomical reference, the tracer uptake cannot be localized. For this display, a PET flow tracer study (c) was aligned with the MR image (a) and the co-registration matrix was subsequently applied to the FIAU data (b) (reproduced with permission from reference [58])
Conclusion
We reviewed the studies in which both MRI and PET have been used in cardiovascular applications. Historically, validation studies in which the replacement of one imaging modality with the other represented the majority of the examples. However, these studies also demonstrated several scenarios where each modality had unique strengths and that they were shown to be able to complement each other. Thus, combination of PET and MRI providing fast imaging protocols without additional radiation exposure appears to be a very attractive imaging solution for evaluation of several cardiovascular diseases. Whether or not the complex technical instrumentation and the expected high costs will offset the advantages of combined PET and MRI remains to be seen.
References
Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, et al. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). J Am Coll Cardiol 2003;42:1318–33.
Eisner RL, Patterson RE. Attenuation correction for stress and rest PET 82Rb myocardial perfusion images. J Nucl Med 2007;48:1912–3.
Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol 2002;39:1151–8.
Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J 2005;26:1115–40.
Beyer T, Townsend DW, Brun T, Kinahan PE, Charron M, Roddy R, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med 2000;41(8):1369–79.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation 2003;108:1664–72.
Martinez-Moller A, Zikic D, Botnar RM, Bundschuh RA, Howe W, Ziegler SI, et al. Dual cardiac-respiratory gated PET: implementation and results from a feasibility study. Eur J Nucl Med Mol Imaging 2007;34:1447–54.
Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 2002;18:539–42.
Makela T, Clarysse P, Sipila O, Pauna N, Pham QC, Katila T, et al. A review of cardiac image registration methods. IEEE Trans Med Imaging 2002;21:1011–21.
Sinha S, Sinha U, Czernin J, Porenta G, Schelbert HR. Noninvasive assessment of myocardial perfusion and metabolism: feasibility of registering gated MR and PET images. AJR Am J Roentgenol 1995;164:301–7.
Mäkela T, Clarysse P, Lötjönen J, Sipilä O, Lauerma K, Hänninen H, et al. A new method for the registration of cardiac PET and MR images using deformable model based segmentation of the main thorax structures. In: Niessen W, Viergever M, editors. Medical image computing and computer-assisted intervention – MICCAI 2001. Lecture Notes in Computer Science, vol. 2208. 4th International Conference, Utrecht, The Netherlands, October 14–17, 2001. Heidelberg: Springer; 2001. p. 557–64.
Martinez-Moller A, Souvatzoglou M, Navab N, Schwaiger M, Nekolla SG. Artifacts from misaligned CT in cardiac perfusion PET/CT studies: frequency, effects, and potential solutions. J Nucl Med 2007;48:188–93.
Gould KL, Pan T, Loghin C, Johnson NP, Guha A, Sdringola S. Frequent diagnostic errors in cardiac PET/CT due to misregistration of CT attenuation and emission PET images: a definitive analysis of causes, consequences, and corrections. J Nucl Med 2007;48:1112–21.
Souvatzoglou M, Bengel F, Busch R, Kruschke C, Fernolendt H, Lee D, et al. Attenuation correction in cardiac PET/CT with three different CT protocols: a comparison with conventional PET. Eur J Nucl Med Mol Imaging 2007;34:1991–2000.
Bluemke DA, Achenbach S, Budoff M, Gerber TC, Gersh B, Hillis LD, et al. Noninvasive coronary artery imaging: magnetic resonance angiography and multidetector computed tomography angiography: a scientific statement from the american heart association committee on cardiovascular imaging and intervention of the council on cardiovascular radiology and intervention, and the councils on clinical cardiology and cardiovascular disease in the young. Circulation 2008;118:586–606.
Yoshinaga K, Chow BJ, Williams K, Chen L, deKemp RA, Garrard L, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol 2006;48:1029–39.
Marwick TH, Shan K, Patel S, Go RT, Lauer MS. Incremental value of rubidium-82 positron emission tomography for prognostic assessment of known or suspected coronary artery disease. Am J Cardiol 1997;80:865–70.
Sawada S, Muzik O, Beanlands RS, Wolfe E, Hutchins GD, Schwaiger M. Interobserver and interstudy variability of myocardial blood flow and flow-reserve measurements with nitrogen 13 ammonia-labeled positron emission tomography. J Nucl Cardiol 1995;2:413–22.
Kaufmann PA, Gnecchi-Ruscone T, Yap JT, Rimoldi O, Camici PG. Assessment of the reproducibility of baseline and hyperemic myocardial blood flow measurements with 15O-labeled water and PET. J Nucl Med 1999;40:1848–56.
El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med 2005;46:1264–71.
Schwaiger M, Melin J. Cardiological applications of nuclear medicine. Lancet 1999;354:661–6.
Manning WJ, Atkinson DJ, Grossman W, Paulin S, Edelman RR. First-pass nuclear magnetic resonance imaging studies using gadolinium-DTPA in patients with coronary artery disease. J Am Coll Cardiol 1991;18:959–65.
Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease: a meta-analysis. J Am Coll Cardiol 2007;50:1343–53.
Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J 2008;29:480–9.
Schwitter J, DeMarco T, Kneifel S, von Schulthess GK, Jorg MC, Arheden H, et al. Magnetic resonance-based assessment of global coronary flow and flow reserve and its relation to left ventricular functional parameters: a comparison with positron emission tomography. Circulation 2000;101:2696–702.
Ibrahim T, Nekolla SG, Schreiber K, Odaka K, Volz S, Mehilli J, et al. Assessment of coronary flow reserve: comparison between contrast-enhanced magnetic resonance imaging and positron emission tomography. J Am Coll Cardiol 2002;39:864–70.
Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, et al. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial. The CECaT trial. Health Technol Assess 2007;11:iii–iv, ix-115.
Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, et al. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med 2001;345:1863–9.
Larose E, Yeghiazarians Y, Libby P, Yucel EK, Aikawa M, Kacher DF, et al. Characterization of human atherosclerotic plaques by intravascular magnetic resonance imaging. Circulation 2005;112:2324–31.
Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation 2002;106:296–9.
Libby P. Inflammation in atherosclerosis. Nature 2002;420:868–74.
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation 2002;105:2708–11.
Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature 2008;451:953–7.
Germano G, Kiat H, Kavanagh PB, Moriel M, Mazzanti M, Su HT, et al. Automatic quantification of ejection fraction from gated myocardial perfusion SPECT. J Nucl Med 1995;36:2138–47.
Khorsand A, Graf S, Frank H, Kletter K, Sochor H, Maurer G, et al. Model-based analysis of electrocardiography-gated cardiac (18)F-FDG PET images to assess left ventricular geometry and contractile function. J Nucl Med 2003;44:1741–6.
Schaefer WM, Lipke CS, Nowak B, Kaiser HJ, Reinartz P, Buecker A, et al. Validation of QGS and 4D-MSPECT for quantification of left ventricular volumes and ejection fraction from gated 18F-FDG PET: comparison with cardiac MRI. J Nucl Med 2004;45:74–9.
Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2000;2:271–8.
Bengel FM, Lehnert J, Ibrahim T, Klein C, Bulow HP, Nekolla SG, et al. Cardiac oxidative metabolism, function, and metabolic performance in mild hyperthyroidism: a noninvasive study using positron emission tomography and magnetic resonance imaging. Thyroid 2003;13:471–7.
Schinkel AF, Poldermans D, Elhendy A, Bax JJ. Assessment of myocardial viability in patients with heart failure. J Nucl Med 2007;48:1135–46.
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992–2002.
Klein C, Nekolla SG, Balbach T, Schnackenburg B, Nagel E, Fleck E, et al. The influence of myocardial blood flow and volume of distribution on late Gd-DTPA kinetics in ischemic heart failure. J Magn Reson Imaging 2004;20:588–93.
Klein C, Schmal TR, Nekolla SG, Schnackenburg B, Fleck E, Nagel E. Mechanism of late gadolinium enhancement in patients with acute myocardial infarction. J Cardiovasc Magn Reson 2007;9:653–8.
Klein C, Nekolla SG, Bengel FM, Momose M, Sammer A, Haas F, et al. Assessment of myocardial viability with contrast-enhanced magnetic resonance imaging: comparison with positron emission tomography. Circulation 2002;105:162–7.
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med 2000;343:1445–53.
Kuhl HP, Lipke CS, Krombach GA, Katoh M, Battenberg TF, Nowak B, et al. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography–single photon emission computed tomography imaging protocol. Eur Heart J 2006;27:846–53.
Gerber BL, Rochitte CE, Bluemke DA, Melin JA, Crosille P, Becker LC, et al. Relation between Gd-DTPA contrast enhancement and regional inotropic response in the periphery and center of myocardial infarction. Circulation 2001;104:998–1004.
Schmidt M, Voth E, Schneider CA, Theissen P, Wagner R, Baer FM, et al. F-18-FDG uptake is a reliable predictory of functional recovery of akinetic but viable infarct regions as defined by magnetic resonance imaging before and after revascularization. Magn Reson Imaging 2004;22:229–36.
Higuchi T, Nekolla SG, Jankaukas A, Weber AW, Huisman MC, Reder S, et al. Characterization of normal and infarcted rat myocardium using a combination of small-animal PET and clinical MRI. J Nucl Med 2007;48:288–94.
Haas F, Haehnel CJ, Picker W, Nekolla S, Martinoff S, Meisner H, et al. Preoperative positron emission tomographic viability assessment and perioperative and postoperative risk in patients with advanced ischemic heart disease. J Am Coll Cardiol 1997;30:1693–700.
Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006;113:2733–43.
Bengel FM, Schwaiger M. Assessment of cardiac sympathetic neuronal function using PET imaging. J Nucl Cardiol 2004;11:603–16.
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BL. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J 2008;29:1147–59.
Sasano T, Abraham MR, Chang KC, Ashikaga H, Mills KJ, Holt DP, et al. Abnormal sympathetic innervation of viable myocardium and the substrate of ventricular tachycardia after myocardial infarction. J Am Coll Cardiol 2008;51:2266–75.
Haubner R, Weber WA, Beer AJ, Vabuliene E, Reim D, Sarbia M, et al. Noninvasive visualization of the activated alphavbeta3 integrin in cancer patients by positron emission tomography and [18F]Galacto-RGD. PLoS Med 2005;2:e70.
Higuchi T, Bengel FM, Seidl S, Watzlowik P, Kessler H, Hegenloh R, et al. Assessment of alphavbeta3 integrin expression after myocardial infarction by positron emission tomography. Cardiovasc Res 2008;78:395–403.
Makowski MR, Ebersberger U, Nekolla S, Schwaiger M. In vivo molecular imaging of angiogenesis, targeting {alpha}v{beta}3 integrin expression, in a patient after acute myocardial infarction. Eur Heart J. 2008 Mar 27.
Zhang SJ, Wu JC. Comparison of imaging techniques for tracking cardiac stem cell therapy. J Nucl Med 2007;48:1916–9.
Bengel FM, Anton M, Richter T, Simoes MV, Haubner R, Henke J, et al. Noninvasive imaging of transgene expression by use of positron emission tomography in a pig model of myocardial gene transfer. Circulation 2003;108:2127–33.
Acknowledgements
Dr. Saraste received financial assistance from the EC-FP6-project DiMI (LSHBCT-2005-512146) and the Finnish Foundation for Cardiovascular Research. A. Martinez-Moeller is funded partially by a research grant from Siemens Healthcare, Erlangen, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nekolla, S.G., Martinez-Moeller, A. & Saraste, A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging 36 (Suppl 1), 121–130 (2009). https://doi.org/10.1007/s00259-008-0980-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0980-1