Abstract
Purpose
To compare the prediction of therapeutic hepatic radiation-absorbed dose rates from tracer imaging plus a linearity assumption to estimation based on intra-therapy imaging in 131I metaiodobenzylguanidine (MIBG) therapy of refractory neuroblastoma.
Materials and methods
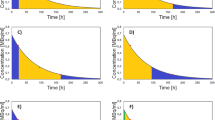
Conjugate-view images of the liver were obtained before therapy for seven patients at seven times after a tracer infusion of 131I MIBG and at three times after the therapy infusion. Measured liver activities were converted to dose-rate estimates. Three statistical models of the rates assuming double exponential dependences on time were examined. One of the three models allowed for a multiplicative correction to the therapeutic late-phase dose-rate amplitude. Results from that model: (1) the tracer prediction of the late-phase absorbed-dose-rate amplitude was a factor of 1.75 times the intra-therapy-estimated value, and (2) the difference between tracer prediction of the radiation-absorbed dose and intra-therapy estimation of it was statistically significant, and (3) the liver radiation-absorbed dose did not reach 30 Gy.
Conclusions
A statistical modeling analysis finds that the radiation-absorbed dose after therapy appears to be lower than that which is predicted from the linear scaling with administered activity of the tracer radiation-absorbed dose. Hepatocyte toxicity is the most likely reason but it is not high enough to produce clinically observable results.



Similar content being viewed by others
References
Brodeur GM, Castleberry RP. Neuroblastoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatrics. Lippincott-Raven: Philadelphia, PA; 1997. p. 761–97.
Matthay KK. Neuroblastoma. In: Pochedly C, editor. Neoplastic diseases in childhood. Chur, Switzerland: Harwood; 1994. p. 735–78.
Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and cis retinoic acid. New Engl J Med 1999;341(16):1165–73.
Ladenstein R, Lasset C, Hartmann O, et al. Impact of megatherapy on survival after relapse from stage 4 neuroblastoma in patients over 1 year of age as diagnosis: a report from the European Group for bone marrow transplantation. J Clin Oncol 1993;11:2330–41.
Ladenstein R, Philip T, Lasset C, et al. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry K. Clin lin Oncol 1998;16:953–65.
Wieland DM, Wu JL, Brown LE, et al. Radiolabeled adrenergic neuron blocking agents: adenomedullary imaging with 131-I-iodobenzylguanidine. J Nucl Med 1980;21:349–53.
McEwan AJ, Shapiro B, Sisson JC, et al. Radioiodobenzylguanidines for the scintigraphic location and therapy of adrenergic tumors. Semin Nucl Med 1985;15(2):132–53.
Hattner RS, Huberty JP, Engelstad BL, et al. Localization of m-iodo (131-I) benzylguanidine in neuroblastoma. Am J Roentegenol 1984;143:373–4.
Matthay KK, DeSantes K, Hasegawa B, Huberty J, Hattner RS, Ablin A, et al. Phase I dose escalation of 131I-metaiodobenzylguanidine with autologous bone marrow support in refractory neuroblastoma. J Clin Oncol 1998;16:229–36.
Yanik GA, Levine JA, Matthay KK, Sisson JC, Shulkin BL, Shapiro B, et al. 131-I-meta-iodobenzylguanidine in combination with myeloablative chemotherapy and autologous stem cell support for the treatment of neuroblastoma. J Clm Oncol. 2002;20(8).
Matthay KK, Panina C, Huberty J, et al. Correlation of tumor and whole body dosimetry with tumor response and toxicity in refractory neuroblastoma treated with 131I-MIBG. J Nucl Med 2001;42:1713–21.
Jacobsson L, Mattsson S, Johansson L, Lindberg S, Fjalling M. Biokinetics and dosimetry of 131-I-metaiodobenzylguanidine (MIBG). Proceeding of Fourth International Radiopharmaceutical Dosimetry Symposium. Oak Ridge, TN. Nov. 5–8, 1985. p. 389–98.
Matthay KK, Tan JC, Villablanca JG, et al. Phase I dose escalation of iodine-131-metaiodobenzylguanidine with myeloablative chemotherapy and autologous stem-cell transplantation in refractory neuroblastoma: a new approaches to Neuroblastoma Therapy Consortium Study. J Clin Oncol 2006;24(3):500–6.
Matthay KK, Quach A, Franc BL, Huberty J, Hawkins RA, Jackson H, et al. 131I-Metaiodobenzylguanidine (131I-MIBG) double infusion with autologous stem cell rescue for neuroblastoma: A New Approaches to Neuroblastoma Therapy (NANT) phase I study. J Clin Oncol 2008; (in press).
Shulkin BL, Sisson JC, Koral KF, et al. Conjugate view gamma camera method for estimating tumor uptake of iodine -131-metaiodobenzylguanidine. J Nucl Med 1988;29:542–8.
Geigy JR, Diem K, Lentner C. Scientific tables. J. R. Geigy Publishers: Basle; 1970.
Cristy M, Eckerman KF. Specific absorbed fractions of energy at various ages from internal photon sources. Oak Ridge National Laboratory ORNL/TM-8381. 1987.
Boeckmann AJ, Beal SL, Sheiner LB. NONMEM users guide. Parts I-VIII. San Francisco CA: UCSF; 2006.
Zasadny KR, Koral KF, Swailem FM. Dead time of an anger camera in dual-energy-window-acquisition mode. Med Phys 1993;20(4):1115–20.
Sisson JC, Avram AM, Lawson SA, Gauger PG, Doherty GM. The so-called stunning of thyroid tissue. J Nucl Med 2006;47(9):1406–12.
Koral KF, Li J, Dewaraja Y, Barrett CL, Regan D, Zasadny KR, et al. 131I anti-B1 therapy/tracer uptake ratio using a new procedure for fusion of tracer images to computed tomography images. Clinical Cancer Research 1999;5:3004–9.
Koral KF, Zasadny KR, Dewaraja Y, Li J, Regan DD, Rommelfanger SG, et al. Update on hybrid conjugate-view SPECT tumor dosimetry and response in 131I -tositumomab therapy of previously-untreated lymphoma patients. J Nucl Med 2003;44(3):457–64.
Dewaraja YK, Kaminski M, Wilderman SJ, Koral K, Kritzman J, Lawson S, et al. Initial results for 3D patient specific dosimetry in 131I tositumomab using dual modality SPECT/CT imaging at multiple times of patients with follicular lymphoma. J Nucl Med 2006;47(5):156P.
Acknowledgements
The research was supported in part by the National Institute of Health grant PO1 CA81403, R21 CA097758, 2MO1 RR0127, as well by donations from the Katie Dougherty Family Foundation, Alex’s Lemonade Stand Foundation, Children’s Neuroblastoma Cancer Foundation, Neuroblastoma Children’s Cancer Society, Pediatric Cancer Research Foundation, and the Evan T. J. Dunbar Neuroblastoma Foundation. No conflict of interest exists for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koral, K.F., Huberty, J.P., Frame, B. et al. Hepatic absorbed radiation dosimetry during I-131 Metaiodobenzylguanadine (MIBG) therapy for refractory neuroblastoma. Eur J Nucl Med Mol Imaging 35, 2105–2112 (2008). https://doi.org/10.1007/s00259-008-0873-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0873-3