Abstract
Purpose
To investigate whether 11C-N-methylspiperone (11C-NMSP) microPET could be used for imaging neural stem cells (NSCs) transplantation in a rat model of traumatic brain injury.
Methods
NSCs were induced to express dopamine receptor type 2 (DRD2), then confirmed by RT-PCR, Western blotting and immunocytochemistry. Eighteen rats were subjected to focal traumatic brain injury in the right parietal lobe and then assigned randomly to the transplantation group and the control group. NSCs labeled with 5-bromo-2-deoxyuridine (BrdU) were transplanted into the cerebral lesion of the transplatation group. MicroPET scan using 11C-NMSP and 18F-FDG were performed to detect the DRD2 expression of transplanted NSCs and the regional glucose metabolism in the cerebral lesion, respectively. Behavioral neurological function of rats were also tested.
Results
Histological analysis identified viable NSCs. Western blotting and immunofluorescence showed high level of NSCs-induced DRD2 expression. Immunostaining demonstrated high levels of survived BrdU+ and DRD2+ donor cells in the cerebral lesion 2 weeks after transplantation. The lesion-to-normal contralateral ratio (L/N ratio) of 11C-NMSP in the cerebral lesion decreased significantly from 97% to 68% after injury and increased dramatically to 137% 1 day after the transplantation and then decreased gradually. Glucose metabolism showed a decrease of 35% in the cerebral lesion 1 day after injury and recovered to 87% 2 weeks after transplantation. The behavioral neurological function of the transplantation group was significantly improved compared with the control group.
Conclusions
This study verified that 11C-NMSP microPET can be used to assess the NSCs-induced DRD2 expression in rat model.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is a major public health problem, especially among male adolescents and young adults aged 15 to 24 years and among elderly people of both sexes 75 years and older. Children aged 5 years and younger are also at high risk for TBI. Its incidence is increasing rapidly in China and other countries [1]. Since injured brain tissue has limited regenerative capacity, the use of stem cells to repair or replace damaged brain tissue became a new and exciting avenue of research [2].
Neural stem cell (NSC) is capable of self-renewal and differentiation into neurons and glias, which participate in repairing damaged neural tissue; thus, it is a promising donor cell source for injury, infarction, and degeneration disease in central nervous system [2]. NSCs persist in the hippocampus, subventricular zone, cortex, and spinal cord, even in the adult [3]. Isolated NSCs are able to proliferate in response to basic fibroblast growth factor or epidermal growth factor, and when the culture conditions are altered, NSCs differentiate into several phenotypes of neurons. Furthermore, neurons derived from NSCs form functional synapses in vitro and in vivo [4]. These results suggest that NSCs have the potential to differentiate into appropriate neurons to form a functional neuronal circuitry [2].
NSC tends to migrate in a long distance and integrate with host cells after transplantation, so it is not easy to differentiate NSC from host cells [5, 6]. In the animal transplantation experiment, NSCs are usually labeled with 5-bromo-2-deoxyuridine (BrdU) or reporter gene [e.g., green fluorescent protein (GFP)] for cell tracking purpose. After transplantation, the NSCs can be detected by immunohistochemistry or fluorescence imaging on the brain tissue slice; however, it is difficult to apply this kind of invasive method in clinical practice. Currently, three methods can be used to noninvasively track stem cells in vivo: magnetic resonance imaging (MRI), in vivo optical imaging, and nuclear medicine [7]. Though MRI has the advantages of a very high spatial resolution and allows repetitive measurements in the same animal, its major shortcoming is the limited number of molecular probes. In addition, it has been demonstrated that the labeling of NSCs with superparamagnetic iron oxide (SPIO) allows in vivo MRI tracking of cells [8, 9]. However, the obvious limitation of this method is that magnetic signal is independent to the living status of donor cells [10]. In another word, if NSCs carrying SPIO died soon in the host brain, SPIO maintains its paramagnetic feature and is still detectable to MRI until SPIO itself was cleaned away. This limitation may lead to an incorrect diagnosis. There is no sufficient evidence supporting that the SPIO existence period just equals to the life span of the donor cell carrying the SPIO. Optical imaging is relatively inexpensive and can be performed repetitively in the same animal but is largely limited to imaging of small animals. Furthermore, optical imaging is impossible to be used for clinical study. Nuclear medicine offers the possibility of monitoring the expression of reporter genes in vivo in humans, as well as in small animals [11]. Positron emission tomography (PET) imaging technology has been dramatically broadening its applications from clinical side to biological research [11, 12]. The goal of microPET is to provide a similar in vivo imaging capability in small animal, so one can transfer knowledge and molecular measurements between species and bring the in-depth understanding gained in mouse models of human disease to the ultimate laboratory setting of the patient [11]. The combination of microPET and reporter gene system enables researchers to track cells bearing reporter genes in vivo in a noninvasive, quantitative, and repetitive way [13].
Previous study reported the utility of two mutated dopamine receptor type 2 (DRD2) genes as PET reporter genes using 3-(2′-[18F]-fluoroethyl)-spiperone (18F-FESP) and 9-(4-[18F]-fluoro-3-hydroxymethylbutyl) guanosine (18F-FHBG) [14]. In general, DRD2 expression is at normal level in NSC; we hypothesized that upregulated DRD2 expression in NSC could be induced and be detected by microPET using 11C-N-methylspiperone (11C-NMSP). Furthermore, we assumed that the metabolic/functional change after DRD2-positive NSC transplantation therapy could be monitored by 18F-fluorodeoxyglucose (FDG) microPET. Thus, in this study, we aimed to evaluate whether microPET can monitor NSC transplantation in a brain-injured rat model.
Materials and methods
Neural stem cell culture
Newborn Sprague–Dawley rats were sacrificed under deep anesthesia with pentobarbital (80 mg/kg, intraperitoneal). Animals were treated according to the guidelines of the University Animal Committee. Sufficient attention was paid to minimizing the suffering of the animals. The hippocampal tissues were dissected from the rat brains and washed in sterile phosphate-buffered saline (PBS, pH 7.4) with 0.6% glucose. Tissue pieces were incubated in 0.05% trypsin (Gibco) with 0.012% EDTA for 15 min at 37°C and then triturated in the same solution using a polished Pasteur pipette. Cell counts showed more than 85% viable cells. Cells were collected by centrifugation and seeded in substrate-free tissue culture flasks at a density of 50,000/mm. The growth medium consisted of Dulbecco’s modified Eagle medium (DMEM)/F12 medium (1:1, Gibco), B27 supplement (1:50, Gibco), human recombinant EGF (20 ng/ml, Sigma), and bFGF (20 ng/ml, Sigma). Cells were incubated in a 37°C incubator with 95% air, 5% CO2, and 100% humidity. Fresh medium was supplemented every 2–3 days. Passaging was carried out every week and consisted of a gentle mechanical dissociation using a polished Pasteur pipette, after which the mixture of small spheres and single cells were re-seeded into fresh medium. Immunocytochemistry for CD133, which is a specific marker of stem cells from many sources, was performed to verify the neural stem cells.
Induction of DRD2 expression and BrdU labeling
Single-cell suspension was prepared as described previously [15] with some modification. Briefly, neurospheres were harvested, incubated in PBS buffer containing 0.05% trypsin and 0.012% EDTA for 20 min at room temperature (RT), and triturated gently twice every 5 min using a fine polished Pasteur pipette. Afterward, the suspension was centrifuged at 1,000 rpm for 5 min to collect the cells. The resuspended cell pellet was exposed to fresh DMEM/F12 medium containing 10% fetal bovine serum and brain-derived neurotrophic factor (BDNF; 20 ng/ml, Sigma), which is sufficient to induce the hippocampal NSC to express DRD2. Cells were seeded in polylysine-coated flasks and incubated at 37°C for 3 days. Then, RT-PCR, Western blotting, and immunocytochemistry were performed to detect the expression of DRD2. BrdU-labeling method was used to identify the existence of NSCs. BrdU was supplemented to the medium everyday. The final concentration of BrdU was 3 μg/ml. BrdU-labeling index of neural stem cells was studied by immunostaining.
Reverse transcriptase PCR (RT-PCR) analysis
Total RNA of neural stem cells was extracted with Trizol reagent (Invitrogen). The forward primer sequence was 5′-CTCCTGCCCACTGCTCTT 3′ and the reverse, 5′-ATCCATTCTCCGCCTGTT 3′. Single-step RT-PCR (Promega Access RT-PCR kit) was performed with these primers and RNA template. Reverse transcription was performed at 45°C for 50 min. PCR reaction program was described as follows: predenature: 4 min at 94°C; denature: 40 s at 94°C; annealing: 1 min at 60°C; polymerization: 1 min at 72°C; cycle number: 38; extension: 8 min at 72°C. The production was analyzed with electrophoresis.
Western blot analysis
To analyze DRD2 expression, NSCs and baseline cells (without DRD2 induction) were washed with ice-cold PBS. Add 200 μl lysis buffer containing 50 mM Tris–HCl (pH 7.5), 100 mM NaCl, 1% SDS, 20 mM EDTA. After sonication for 30 s, 40 μl of sample buffer (200 mM Tris–HCl, pH 6.8, 10% SDS, 25% beta-mercaptoethanol, 25% glycerol, and 0.01% bromophenol blue) was added. Samples were boiled for 5 min, and 20 μg aliquots was subjected to SDS-PAGE on 10% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore). After blocking with 10% nonfat dry milk in Tris-buffered saline containing 0.05% Tween-20 overnight at 4°C, blots were probed with anti-DRD2 antibody (1:250, Santa Cruz) for 1.5 h at RT and then incubated with horseradish peroxidase (HRP)-linked rabbit anti-goat IgG, diluted to 1:5,000 for 1 h at RT. Immunoreactive bands were visualized with an enhanced chemiluminescence system (ECL, Santa Cruz).
Immunocytochemistry
Immunostaining was performed as previously described [15], with some modifications. Briefly, the cells attached to the coverslips were washed three times with PBS and fixed using 4% paraformaldehyde in PBS solution for 30 min. The cells were then permeabilized with cold 0.5% Triton-X-100 in PBS for 10 min at RT and washed with PBS. PBS was supplemented with 10% FBS, and 5% nonfat milk was used as a blocking solution (30 min, 37°C). Then, the cells were incubated at 4°C overnight in primary goat anti-DRD2 antibody (Santa Cruz; dilution 1:250), goat anti-CD133 antibody (Santa Cruz; dilution 1:200), or mouse anti-BrdU antibody (Santa Cruz; dilution 1:200). After being washed with PBS, the cells were incubated at 37°C for 30 min with FITC-labeled rabbit anti-goat IgG, HRP-labeled rabbit anti-goat, or HRP-labeled goat anti-mouse IgG antibodies, respectively. As for the HRP-labeled secondary antibodies, cells were stained with diaminobenzidine (DAB) and counterstained with hematoxylin to visualize cell nuclei. Triton-X-100 treatment was neglected in CD133 immunostaining. Additionally, as for BrdU, cells should be incubated in 1 N HCl for 30 min at 95°C before blockage.
Animal experiment
Eighteen Sprague–Dawley rats were assigned randomly to the transplantation group (n = 10) or the control group (n = 8). Rat models of right parietal lobe brain injury were made as described previously [16]. Briefly, rat models of focal brain trauma were made following Feeney’s method. After anesthesia with pentobarbital (40 mg/kg, intraperitoneal), the rat was fixed on a stereotactic apparatus. A piece of right parietal bone was removed to form a cranial window. Then, the exposed dura of the right parietal lobe was hit directly by a falling object with an impact force that was 1,250 g cm−2. One day later, 20 μl BrdU-labeled neural stem cell suspension (at a density of 5 × 107/ml) was injected into the traumatic lesion through a fine needle. For the control group, animals were subjected to traumatic brain injury in the same way, and the same amount of saline instead of cell suspension was injected into the lesion. Aseptic technique principle was strictly followed in animal experiments. Two weeks later, after microPET imaging, animals were sacrificed under deep anesthesia with pentobarbital (80 mg/kg, intraperitoneal), and then the brain tissues were dissected and studied in vitro. Animal care and euthanasia were performed with the approval of the Zhejiang University Animal Committee.
MicroPET imaging
11C-NMSP was synthesized with a simple modification from the original method of DonaldBurns [17]. At different time course (prior to trauma, 1 day post-trauma, and 1, 7, and 14 days post-transplantation), 11C-NMSP microPET scans were conducted to detect the DRD2 expression in the brain. The regional glucose metabolism of the brain lesion was evaluated in vivo by 18F-FDG microPET imaging at the beginning and the end of the animal experiment. Briefly, the rat was anesthetized with pentobarbital (40 mg/kg, intraperitoneal) and injected intravenously with 0.5 mCi 11C-NMSP or 0.1 mCi 18F-FDG through the tail vein. After an uptake period of 20 min, the animal was placed in a spread prone position and scanned with the microPET R4 (Concorde Microsystems, Knoxville, TN, USA), which consists of a 15-cm diameter ring of 96 position-sensitive γ-ray scintillation detectors, providing a 10.8-cm transaxial and a 7.8-cm axial field of view, with image resolution <1.8 mm. A 10-min static acquisition was performed in three-dimensional mode, and images were reconstructed by a maximum-a-posteriori probability algorithm. The corrections for dead time, random scattering, and attenuation were performed. For semi-quantitative evaluation, region of interest (ROI) method was used for the evaluation of regional uptake change of PET tracer. ROI was drawn around the area of hypometabolism in the right injured parietal cortex on the 18F-FDG microPET image, then the ROI was duplicated on the same slice level of the NMSP microPET image of the same rat. Same position was used for series of microPET scans in order to avoid any possible spatial mismatch and obtain correct slice for analysis. The mean activities of ROIs in the injured right parietal cortex and the homologous contralateral region were calculated. The lesion-to-normal homologous contralateral ratio (L/N ratio) was used for the semi-quantitative analysis. L/N ratio was calculated using the following formula: L/N ratio = mean counts per pixel of lesion region of interest/mean counts per pixel of normal homologous contralateral region of interest.
Assessment of neurological motor function
Neurological motor function was evaluated 2 week after cell transplantation. Evaluation of motor function was performed by blinded, trained observers who used standardized, well-established tests of balance and vestibulomotor function (tiltboard tests). The rat was place on the end of the tiltboard horizontally. The board (30 cm in width) had a coarse rubber surface and tilted with an initial angle of 30° and an acceleration of 1°/s. The trial was terminated if the animal fell off the board. Two trials were performed at intervals of 5 min each, and the critical angles (in degrees) of both trials were averaged.
Immunohistochemistry
Paraffin sections of the brain lesions were hydrated, immersed in a solution of 3% H2O2 in PBS for 10 min, and blocked with rabbit serum for 1 h. Then, the sections were incubated with anti-DRD2 antibody (dilution 1:250) or anti-BrdU antibody (dilution 1:200) for 2 h at 37°C, followed by incubation with HRP-labeled secondary antibodies for 1 h at 37°C. The result was visualized using DAB as the chromagen for 5 min.
Statistical analysis
Data were expressed as means ± SD. Student’s t test, one-way analysis of variance, or X 2 test was used where appropriate. p < 0.05 was accepted as statistically significant.
Results
NSC induced DRD2 expression
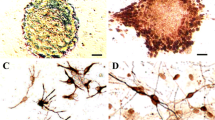
Neural stem cell colonies consisting of four to eight cells emerged in 3 days of primary culture in the serum-free medium. The colonies expanded to more than 100 cells called “neurospheres”. The neurospheres reached about 0.5 mm in diameter 1 week in the primary culture. Thereafter, the neurospheres were passaged. Single cells and small spheres in the subculture aggregated to form neurospheres again and continued to proliferate. Most cells in the neurospheres were CD133 positive, as demonstrated by immunocytochemistry (Fig. 1a). Then, neural stem cell suspension was prepared, seeded in the polylysine-coated flasks, and cultured as monolayer in the medium containing 10% serum and BDNF (20 ng/ml) for 3 days to induce expression of DRD2. RT-PCR revealed abundant transcription of DRD2 (Fig. 1b). Similarly, Western blotting (Fig. 1c) and immunocytochemistry (Fig. 1e) also demonstrated significantly higher level of DRD2 expression in neural stem cells in comparison with baseline cells (Fig. 1d). The stem cells were labeled with BrdU before transplantation. The BrdU-labeling index was about 96% (Fig. 1f).
Preparation of DRD2-positive NSCs. a Most cells in the neurospheres were CD133 positive (immunocytochemistry, magnification ×200). b RT-PCR expanded a clear band of about 300 bp. c Western blotting demonstrated high-level DRD2 expression in NSCs with DRD2 induction. d Western blotting demonstrated general-level DRD2 expression in NSCs without DRD2 induction. e NSCs were induced to express DRD2 (immunofluorescence, magnification ×200). f NSCs were labeled with BrdU before transplantation; the BrdU-labeling index was about 96% (immunocytochemistry, magnification ×400)
MicroPET monitoring of NSC-induced DRD2 expression
11C-NMSP is a specific PET tracer for the detection of DRD2 expression [18]. In the 11C-NMSP microPET image, the striaturn was clearly visualized with good striatum-to-extrastriatal background contrast in normal rat brain (Fig. 2a). Moreover, the microPET image showed that the 11C-NMSP uptake of the normal parietal cortex was symmetrical, which was much lower than that of the striatum (Fig. 2a).
Typical examples of 11C-NMSP microPET imaging in various conditions. Coronal and axial sections of rat brain are shown. White cross markers in the coronal and axial sections indicate the same position in a scan. a Typical image of 11C-NMSP microPET in normal rat brain showing high 11C-NMSP accumulation in striatum. b L/N ratio of 11C-NMSP decreased after traumatic brain injury. c High accumulation of 11C-NMSP indicated the existence of transplanted DRD2-positive NSCs
After traumatic brain injury, the L/N ratio decreased to 68 ± 12% (Fig. 2b and 3). One day after NSC transplantation, however, the L/N ratio increased dramatically to 137 ± 19%, while that of the control group remained 69 ± 10% (p < 0.05, Fig. 3). On the microPET image, a high accumulation of 11C-NMSP was found, indicating the existence of transplanted DRD2-positive NSCs (Fig. 2c). On day 7 of post-transplantation, the L/N ratio of the transplantation group was 118 ± 16% and that of the control, 78 ± 12% (p < 0.05, Fig. 3). On day 14 of post-transplantation, the L/N ratio of the transplantation group was 99 ± 15%, while that of the control was 72 ± 10% (p < 0.05). The 11C-NMSP microPET image also revealed the dynamic decreasing changes of tracer uptake of the transplanted DRD2-positive NSCs during a 14-day course.
Semi-quantitative analysis of 11C-NMSP in various conditions. L/N ratio calculated as the ratio of radioactivity in each ROI to radioactivity in corresponding ROI of contralateral hemisphere. 11C-NMSP uptake of focal traumatic lesion in transplantation group was significantly higher in control group (p < 0.05)
Evaluation of therapeutic effect of NSC transplantation
18F-FDG microPET showed a normal glucose metabolism in normal rat brain (Fig. 4a). One day after traumatic brain injury, the L/N ratio of glucose metabolism decreased to 65 ± 13% from the original normal 99 ± 16%, as demonstrated by the hypometabolic area in the right parietal cortex on the microPET image (Fig. 4b). On the day 14 after transplantation (Fig. 4c), the L/N ratio increased to 87 ± 10%, while that of the control still remained at 72 ± 14% (p < 0.05), showing transplanted NSCs conduced the metabolic recovery of the injured area.
Typical examples of 18F-FDG microPET imaging in various conditions. Coronal and axial sections of rat brain are shown. a Typical image of 18F-FDG microPET in normal rat brain. b As shown with arrows, the focal traumatic lesion appeared as a hypometabolic area in the right parietal cortex. c The regional glucose metabolism of the focal traumatic lesion recovered 14 days after NSCs transplantation
Pathological analysis showed no significant inflammation in the cerebral lesion.
Furthermore, functional recovery measured by the neurological motor function test showed significant improvement in the transplantation group, compared with that of the control group (critical angle 56.5 ± 6.6° vs 47.4 ± 4.2°, p < 0.05).
Immunohistochemistry
Immunohistochemical studies showed that the cells appeared small and round morphologically, with a large nuclear-cytoplasmic ratio (Fig. 5a). Many BrdU- and DRD2-positive cells existed in the cerebral lesion (Fig. 5b and c), which indicated that a high level of donor neural stem cells survived in the traumatic brain and continually expressed DRD2 within 2 weeks after transplantation.
Immunohistochemical confirmation of NSCs. a Many small, round, or spindle-shaped cells survived in the brain lesion (H&E, magnification ×200). b These small cells were DRD2 positive (immunocytochemistry, magnification ×200). c Nucleus of the BrdU-positive cells were stained with brown (immunocytochemistry, magnification ×400)
Discussion
NSC therapy has been tried to treat neurodegenerative diseases, stroke, and brain injuries in human trials and in experimental animals [19]. Stem cell monitoring is essential for studying the transplanted stem cells in vivo. Although reporter genes such as LacZ or GFP have been used to track transplanted stem cells in animal experiments, biopsy or subject sacrifice was required, and sequential measurement was not possible in the same subject. The fate of transplanted cells in vivo is a vital step in determining the efficacy of the implant. It is necessary to know the important features of cellular implants: cell tracking (are the cells reaching the target tissue?), cell viability (once they get there are they alive?), and cell function (if they are alive are they actually functioning?). To monitor the transplanted stem cells repeatedly, molecular imaging is required to enable noninvasive examination of the migration and possibly proliferation of stem cells in vivo.
PET offers several advantages over SPECT, MRI, and optical approaches for imaging reporter gene expression. PET is able to measure biological processes at very low concentrations. The mass of radiotracer injected is small and generally does not impact the biological system under study. Technological developments of PET have led to the implementation of specialized systems such as microPET for small animal imaging, with much higher spatial resolution (<2 mm), which has dramatically advanced the field of cell tracking in animal models in vivo. Various gene expression imaging systems have been developed for PET using various reporter gene systems, including herpes simplex virus type 1 thymidine kinase (HSV1-TK), cytosine deaminase, and DRD2 and sodium–iodide symporter [20, 21].
Su et al. reported a methodology for tracking and estimation of cells using PET molecular imaging system (HSV1-sr39TK/18F-FHBG) and successfully determined the parameters for enumeration of primary murine T-lymphocytes expressing sr39TK [22]. A procedure to image reporter gene expression in mice with PET, using the DRD2 as a reporter gene and 3-(2-[18F]fluoroethyl)-spiperone as a reporter probe has been reported by MacLaren et al. [23]. This study demonstrated that tumors expressing the transfected DRD2 reporter gene retain substantially more FESP than control tumors in mouse model, showing that DRD2/18F-FESP is a valuable technique to monitor in vivo the expression from both gene therapy vectors and transgenes. Furthermore, the same research group investigated reporter gene expression of HSV1-tk (18F-FHBG probe) and DRD2 (18F-FESP probe) from the proximal and distal positions of a bicistronic message following systemic administration of an adenovirus-based gene-delivery system and concluded that it is possible, in living mice, to investigate noninvasively and to measure quantitatively and repeatedly correlated expression of two coding regions from a bicistronic transcription unit over a 3-month period following adenovirus delivery [24]. The findings extended microPET and molecular imaging with new quantitative cell biology tools. Although HSV1-TK and its mutant are the most popular reporter genes for microPET imaging, they are unsuitable for tracing neural stem cells in the brain because the substrates (probes) for HSV1-TK PET reporter genes can not cross the intact blood–brain barrier [25], thus preventing the easy utilization of the HSV1-tk PET reporter gene systems for in vivo imaging of the brain. On the other hand, DRD2 reporter gene/18F-FESP reporter probe system as a promising technique for monitoring gene therapy has been investigated in tumor animal model [24]; however, until now, there is no study of using DRD2 as reporter on stem cell therapy monitoring.
In this study, our hypothesis was that hippocampal NSC could be induced to express DRD2 spontaneously, and NSC-induced DRD2 expression would be monitored by 11C-NMSP microPET. As a result, our study shows that microPET can provide a noninvasive in vivo imaging for monitoring transplanted NSCs in a rat model of traumatic brain injury, and NSC transplantation promotes functional recovery in 2 weeks. As we know, the expression of the HSV1-TK enzyme, in the absence of exogenous acycloguanosine substrates, has little or no effect on cells and tissues. In contrast, ectopic expression of the wild-type DRD2 gene, either in transgenic animals or in gene therapy delivery vehicles, might have physiological consequences as a result of interactions with endogenous ligands. Therefore, we tried to induce neural stem cells to express DRD2 spontaneously rather than by gene transduction strategy. Since the hippocampus is enriched in neural stem cells, which would continuously migrated into the granule cell layer [26, 27] and differentiated into DRD2-positive neurons (Fig. 6), we deduced that hippocampal stem cells might be induced to express DRD2 in vitro if certain conditions were given transiently for differentiation. Thus, neural stem cells were cultured as monolayer in serum-containing medium supplemented with sufficient BDNF, a vital factor in the survival and neurogenesis in hippocampus. Interestingly, most neural stem cells derived from hippocampus were induced to express DRD2 in merely 3 days. This population was successfully monitored with 11C-NMSP microPET. Furthermore, we found that L/N ratio of 11C-NMSP decreased in function of time in the transplanted group. This finding is probably due to the loss of viable NSC amount, which caused the loss of DRD2 expression. Thus, this finding indicated that repeated administration of NSCs should be a potential therapeutic option.
To our knowledge, this is the first study on NSC-induced DRD2 expression detected by 11C-NMSP microPET. This study established a relative simple methodology for NSC monitoring.
The biodistribution of NMSP in normal mice and rat have been reported previously [17, 28]. The liver and kidneys were the organs of highest accumulation. Two specific portions of the brain (striatum and cerebellum) had the high accumulation of NMSP during 20–30 min after intravenous injection, showing that the percent injected dose per gram in the striatum was 4.89 and the striatum-to-cerebellum ratio, based on percent dose per gram of each organ, was 20.1, and striatum-to-frontal cortex ratio was 1.3, showing a relative low biodistribution of 11C-NMSP in frontal cortex of brain. In our present study, it is consistent with the previous study. DRD2 levels in cerebral cortex are very difficult to detect; only very sensitive autoradiographic assays have found very low levels of DRD2s in cerebral cortex [29]. This means the potential of 11C-NMSP to identify the high DRD2 expression from the transplanted NSCs in the regions of cerebral cortex.
18F-FDG microPET is sensitive to metabolic changes that occur after traumatic brain injury. Longitudinal 18F-FDG microPET studies conducted concurrently with behavioral studies revealed a significant correlation between duration of metabolic change and traumatic brain injury, illustrating the novel opportunity and value of using in vivo molecular imaging within the same animals to examine therapeutic relationship [30]. In the present study, 18F-FDG microPET was performed before and after NSC transplantation and showed significant metabolic recovery after NSC transplantation, together with the recovery of behavioral neurological and motor function. The combination of 11C-NMSP and 18F-FDG might be a potential approach to evaluate neural stem cell treatment.
Tracer uptake was evaluated by L/N ratio in this study. It has been shown that semi-quantitative analysis by SUV is not as reliable as the tumor-to-liver ratio in evaluation of tumor response to fluorouracil in colorectal cancer liver metastases [31]. This is indicative of the problems of introducing extra measurement variables when trying to establish a semi-quantitative method. Inadequate injection with extravasation of some of the tracer at the time of intravenous administration most likely led to an underestimate of the lesion SUV. This problem did not affect the lesion-to-normal background ratio, because it is purely a ratio of the observed radioactivity in the two ROIs; therefore, injected activity is irrelevant. And as a further potential confounding factor for SUV calculation of tracer, a competing organ could take up a greater proportion of the injected tracer dose, leaving less for the lesion. If this is present only on the pre- or post-transplantation scan, it may result in a misinterpretation of the tumor response. Furthermore, the half life of 11C is too short that any delay in the process of anesthesia, administration of tracer would cause the absolute quantification. On the other hand, the relative radioactivity was valid and effective to evaluate the stem cell therapy because the brain is generally symmetric in DRD2 receptor expression.
Recent clinical PET study reported that hypometabolism of the traumatic or ischemic brain injury recovered after neural stem cell transplantation [32]. However, there is no direct evidence that neural stem cell graft itself rather than other related factors contributed to the metabolic recovery. The findings of our study provided a dynamic reflection of the neural stem cell’s living status in the brain lesion, which showed that the stem cell grafts survived the brain trauma and expressed DRD2. As a result, the lesion metabolism, behavioral, neurological, and motor function were significantly improved after NSC transplantation. Therefore, the present results strongly suggest that, when clinically applied, this approach may provide important information for assessing the effects of NSC transplantation therapy on traumatic brain injury.
Conclusion
Transplanted NSC-induced DRD2 expression can be monitored in the rat model of traumatic brain injury by microPET, and NSC promotes functional recovery. In vitro expandable NSCs may constitute a good source for neural transplantation. The combination of 11C-NMSP and 18F-FDG microPET might be a potential approach for the evaluation of neural stem cell treatment.
References
Sosin DM, Sniezek JE, Waxweiler RJ. Trends in death associated with traumatic brain injury, 1979 through 1992: success and failure. JAMA. 1995;273:1778–80.
Mayhall EA, Paffett-Lugassy N, Zon LI. The clinical potential of stem cells. Curr Opin Cell Biol. 2004;16(6):713–20.
Temple S, Alvarez-Buylla A. Stem cells in the adult mammalian central nervous system. Curr Opin Neurobiol. 1999;9:135–41.
Price J, Williams BP. Neural stem cells. Curr Opin Neurobiol. 2001;11:564–7.
Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–70.
Nelson PT, Kondziolka D, Wechsler L, et al. Clonal human (hNT) neuron grafts for stroke therapy: neuropathology in a patient 27 months after implantation. Am J Pathol. 2002;160:1201–6.
Blasberg RG, Gelovani J. Molecular-genetic imaging: a nuclear medicine-based perspective. Mol Imaging. 2002;1:280–300.
Modo M, Mellodew K, Cash D, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004;21:311–7.
Zhu JH, Zhou LF, Ge F, Wu X. Tracking neural stem cells in patients with brain trauma. New Engl J Med. 2006;12:2376–8.
Arbab AS, Liu W, Frank JA. Cellular magnetic resonance imaging: current status and future prospects. Expert Rev Med Devices. 2006;3:427–39.
Phelps M. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–81.
Tian M, Zhang H, Nakasone Y, Mogi K, Endo K. Expression of Glut-1 and Glut-3 in untreated oral squamous cell carcinoma compared with FDG accumulation in a PET study. Eur J Nucl Med Mol Imaging. 2004;31:5–12.
Wu JC, Inubushi M, Sundaresan G, Schelbert HR, Gambhir SS. Positron emission tomography imaging of cardiac reporter gene expression in living rats. Circulation 2002;106:180–3.
Liang Q, Satyamurthy N, Barrio JR, et al. Noninvasive, quantitative imaging in living animals of a mutant dopamine D2 receptor reporter gene in which ligand binding is uncoupled from signal transduction. Gene Ther. 2001;8:1490–8.
Zheng X, Shen S, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67:3691–7.
Zheng XS, Yang XF, Liu WG, Pan DS, Hu WW, Li G. Transplantation of neural stem cells into the traumatised brain induces lymphocyte infiltration. Brain Injury 2007;21:275–8.
DonaldBurns H, Dannals RF, Langstrom B, et al. (3-N-[11C]Methyl) Spiperone, a ligand binding to dopamine receptors: radiochemical synthesis and biodistribution studies in mice. J Nuci Med. 1984;25:1222–7.
Wagner H, Burns D, Dannals R, et al. Imaging dopamine receptors in the human brain by positron tomography. Science 1986;221:1264–6.
Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000;3:537–44.
Gambhir SS, Barrio JR, Herschman HR, Phelps ME. Assays for noninvasive imaging of reporter gene expression. Nucl Med Biol. 1999;26:481–90.
Gambhir SS, Herschman HR, Cherry SR, et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–38.
Su H, Forbes A, Gambhir SS, Braun J. Quantitation of cell number by a positron emission tomography reporter gene strategy. Mol Imaging Biol. 2004;6:139–48.
MacLaren DC, Gambhir SS, Satyamurthy N, et al. Repetitive, non-invasive imaging of the dopamine D2 receptor as a reporter gene in living animals. Gene Therapy. 1999;6:785–91.
Liang Q, Gotts J, Satyamurthy N, et al. Noninvasive, repetitive, quantitative measurement of gene expression from a bicistronic message by positron emission tomography, following gene transfer with adenovirus. Mol Therapy. 2002;6:73–82.
Jacobs A, Braunlich I, Graf R, et al. Quantitative kinetics of [124I]FIAU in cat and man. J Nucl Med. 2001;42:467–75.
Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H. Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys. Mol Cell Neurosci. 2003;23:292–301.
Limke TL, Cai J, Miura T, Rao MS, Mattson MP. Distinguishing features of progenitor cells in the late embryonic and adult hippocampus. Dev Neurosci. 2003;25:257–72.
Lyon RA, Titeler M, Frost JJ, et al. 3H-3-N-Methylspiperone labels D2 dopamine receptors in basal Ganglia and S2 serotonin receptors in cerebral cortex. J Neurosci. 1986;6:2941–9.
Martres MP, Bouthenet ML, Sales N, Sokoloff P, Schwartz JC. Widespread distribution of brain dopamine receptors evidenced with 1251-sulpride, a highly selective ligand. Science 1985;228:752–5.
Moore AH, Osteen CL, Chatziioannou AF, Hovda DA, Cherry SR. Quantitative assessment of longitudinal metabolic changes in vivo after traumatic brain injury in the adult rat using FDG-microPET. J Cereb Blood Flow Metab. 2000;20:1492–501.
Findlay M, Young H, Cunningham D, et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol. 1996;14:700–8.
Luan Z, Yin GC, Hu XH, et al. Treatment of an infant with severe neonatal hypoxic–ischemic encephalopathy sequelae with transplantation of human neural stem cells into cerebral ventricle. Zhonghua Er Ke Za Zhi. 2005;43:580–3.
Acknowledgments
This study was supported by the grants from the National Science Foundation of China (NSFC) (No. 30672396), the Natural Science Foundation of Zhejiang Province (No. R205066), and a key project grant from the Ministry of Science and Technology of China (MOST; No. 2006DFB32940).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hong Zhang and Xuesheng Zheng contributed to this study equally.
Rights and permissions
About this article
Cite this article
Zhang, H., Zheng, X., Yang, X. et al. 11C-NMSP/18F-FDG microPET to monitor neural stem cell transplantation in a rat model of traumatic brain injury. Eur J Nucl Med Mol Imaging 35, 1699–1708 (2008). https://doi.org/10.1007/s00259-008-0835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0835-9