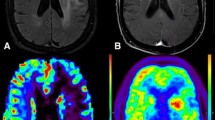
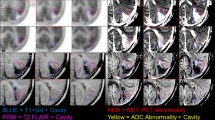
Abstract
Purpose
The rationale of this study was to investigate the feasibility of three-dimensional (3D) methods to analyze 18F-fluoro-deoxy-glucose (FDG) uptake in children with anaplastic astrocytoma (AA) in a multi-institutional trial, to compare 3D and two-dimensional (2D) methods and explore data associations with progression-free survival (PFS).
Methods
3D tumor volumes from pretreatment MR images (fluid attenuation inversion recovery and postgadolinium) of children with recurrent AA on a phase I trial of imatinib mesylate were coregistered to FDG positron emission tomography (PET) images. PET data were normalized. Four metrics were defined: the maximum ratio (maximum pixel value within the 3D tumor volume, normalized), the total ratio (cumulative pixel values within the tumor volume, normalized) and tumor mean ratio (total pixel value divided by volume, normalized). 2D analysis methods were compared. Cox proportional hazards models were used to estimate the association between these methods and PFS.
Results
Strongest correlations between 2D and 3D methods were with analyses using postcontrast T1 images for volume of interest (VOI). The analyses suggest 3D maximum tumor and mean tumor ratios, whether normalized by gray matter or white matter, were associated with PFS.
Conclusions
This study of a series of pretreatment AA patients suggests that 3D PET methods using VOIs based on postcontrast T1 correlate with 2D methods and are related to PFS. These methods yield an estimate of metabolically active tumor burden and may add prognostic information after tumor grade is determined. Future rigorous multi-institutional protocols with larger numbers of patients will be required for validation.



Similar content being viewed by others
References
Barentsz J, Takahashi S, Oyen W, Mus R, De Mulder P, Reznek R, et al. Commonly used imaging techniques for diagnosis and staging. J Clin Oncol 2006;24:3234–44.
Ell PJ. The contribution of PET/CT to improved patient management. Br J Radiol 2006;79:32–6.
Shields AF. Positron emission tomography measurement of tumor metabolism and growth: its expanding role in oncology. Mol Imaging Biol 2006;8:141–50.
Wong TZ, van der Westhuizen GJ, Coleman RE. Positron emission tomography imaging of brain tumors. Neuroimaging Clin N Am 2002;12:615–26.
Borgwardt L, Hojgaard L, Carstensen H, Laursen H, Nowak M, Thomsen C, et al. Increased fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG) uptake in childhood CNS tumors is correlated with malignancy grade: a study with FDG positron emission tomography/magnetic resonance imaging coregistration and image fusion. J Clin Oncol 2005;23:3030–7.
Pollack IF, Jakacki RI, Blaney SM, Hancock ML, Kieran MW, Phillips P, et al. Phase I trial of imatinib in children with newly diagnosed brainstem and recurrent malignant gliomas: a Pediatric Brain Tumor Consortium report. Neuro-oncology 2007;9:145–60.
Poussaint TY, Phillips PC, Vajapeyam S, Fahey FH, Robertson RL, Osganian S, et al. The Neuroimaging Center of the Pediatric Brain Tumor Consortium-collaborative neuroimaging in pediatric brain tumor research: a work in progress. Am J Neuroradiol 2007;28:603–7.
West J, Fitzpatrick JM, Wang MY, Dawant BM, Maurer CR Jr., Kessler RM, et al. Comparison and evaluation of retrospective intermodality brain image registration techniques. J Comput Assist Tomogr 1997;21:554–66.
Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, et al. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 1995;195:47–52.
Di Chiro G, Hatazawa J, Katz DA, Rizzoli HV, De Michele DJ. Glucose utilization by intracranial meningiomas as an index of tumor aggressivity and probability of recurrence: a PET study. Radiology 1987;164:521–6.
Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F] fluorodeoxyglucose and positron emission tomography. Neurology 1982;32:1323–9.
Patronas NJ, Di Chiro G, Kufta C, Bairamian D, Kornblith PL, Simon R, et al. Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg 1985;62:816–22.
Padma MV, Said S, Jacobs M, Hwang DR, Dunigan K, Satter M, et al. Prediction of pathology and survival by FDG PET in gliomas. J Neurooncol 2003;64:227–37.
De Witte O, Levivier M, Violon P, Salmon I, Damhaut P, Wikler D Jr., et al. Prognostic value positron emission tomography with [18F]fluoro-2-deoxy-D-glucose in the low-grade glioma. Neurosurgery 1996;39:470–6. discussion 76–7.
De Witte O, Lefranc F, Levivier M, Salmon I, Brotchi J, Goldman S. FDG-PET as a prognostic factor in high-grade astrocytoma. J Neurooncol 2000;49:157–63.
Salman U, Martin C, Hammond L, Chintapalli K, Denis L, Kuhn J, et al. 9:15–9:30. Use of a Spherical 3-D Blob Analysis Program as a Method of Determination of Standardized Uptake Value (SUV) for Following Tumor Response to Chemotherapeutic Agents (CTA). Clin Positron Imaging 2000;3:152.
Paldino M, Barboriak D, Wong TZ, Reardon D, Friedman H. Correlation of registered quantitative 18-F-FDG-PET and contrast-enhanced MR imaging findings with survival in patients undergoing intracavitary radiation therapy for high grade glioma. In: RSNA 2006. Chicago, IL; 2006.
Acknowledgments
This work was supported in part by NIH grant U01 A81457 for the Pediatric Brain Tumor Consortium (PBTC), The Pediatric Brain Tumor Foundation of the United States (PBTFUS), and American Lebanese Syrian Associated Charities. We acknowledge the site PET physicians including Dr. Abass Alavi (Children’s Hospital of Philadelphia), Dr. Randall Hawkins (UCSF), Dr. James Mountz (Children’s Hospital of Pittsburgh), Dr. Terence Wong (Duke), Dr. Satoshi Minoshima (Seattle Children’s Hospital), Dr. David Earl-Graef (Children’s National), Dr. Stewart Spies (Children’s Memorial), Dr. John Butman (NIH), and Dr. Barry Shulkin (St. Jude Children’s Research Hospital).
Conflict of interest statement
There are no financial disclosures or other relationships that would create a conflict of interest for the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, G., Fahey, F.H., Treves, S.T. et al. Exploratory evaluation of two-dimensional and three-dimensional methods of FDG PET quantification in pediatric anaplastic astrocytoma: a report from the Pediatric Brain Tumor Consortium (PBTC). Eur J Nucl Med Mol Imaging 35, 1651–1658 (2008). https://doi.org/10.1007/s00259-008-0780-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0780-7