Abstract
Purpose
Peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumours with 90Y-DOTATOC and 177Lu-DOTATATE is promising. The kidney is the critical organ and despite renal protection, function loss may become evident years later. The aim of this study was to analyse renal parameters in patients who had undergone dosimetry before PRRT.
Methods
Among those in protocols at our institution, 28 patients were considered: 23 received 90Y-DOTATOC (3.8–29.2 GBq, median 12.2) and five received 177Lu-DOTATATE (20.7–29.2 GBq, median 23.2). Patients were followed up after therapy for creatinine and creatinine clearance loss (CCL) for 3–97 months (median 30). Renal doses and bio-effective doses (BED) were calculated (MIRD, LQ model).
Results
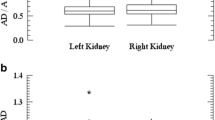
After 90Y-DOTATOC toxicity on creatinine according to NCI criteria occurred in nine cases (seven grade 1, one grade 2, one grade 3), CCL at 1 year was >5% in 12 cases and >10% in eight. A 28-Gy BED threshold was observed in patients with risk factors (mainly hypertension and diabetes), while it was 40 Gy in patients without risk factors. Probably due to the low number of patients, despite the absence of severe toxicity after hyper-fractionated PRRT, clear correlations between fractionation and toxicity could not be found. After 177Lu-DOTATATE, no toxicity occurred in 1–2 year follow-up; CCL at 1 year >5% occurred in three patients and >10% in two.
Conclusions
Our results indicate the importance of clinical screening for risk factors: In this case, a BED <28 Gy is recommended. Fractionation of therapy is important in order to decrease toxicity, and further studies are needed to evaluate its clinical impact.





Similar content being viewed by others
References
de Jong M, Valkema R, Jamar F, Kvols LK, Kwekkeboom DJ, Breeman WA, et al. Somatostatin receptor-targeted radionuclide therapy of tumors: preclinical and clinical findings. Semin Nucl Med 2002;32(2):133–40.
Waldherr C, Pless M, Maecke HR, Schumacher T, Crazzolara A, Nitzsche EU, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq (90)Y-DOTATOC. J Nucl Med 2002;43(5):610–6.
Bodei L, Cremonesi M, Grana C, Rocca P, Bartolomei M, Chinol M, et al. Receptor radionuclide therapy with 90Y-[DOTA]0-Tyr3-octreotide (90Y-DOTATOC) in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2004;31(7):1038–46.
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med 2005;46(Suppl 1):62S–6S.
Valkema R, de Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med 2002;32(2):110–22.
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate in patients with endocrine gastroenteropancreatic tumors. J Clin Oncol 2005;23(12):2754–62.
Bernard BF, Krenning EP, Breeman WA, Rolleman EJ, Bakker WH, Visser TJ, et al. d-lysine reduction of indium-111 octreotide and yttrium-90 octreotide renal uptake. J Nucl Med 1997;38(12):1929–33.
de Jong M, Krenning EP. New advances in peptide receptor radionuclide therapy. J Nucl Med 2002;43(5):617–20.
Paganelli G, Zoboli S, Cremonesi M, Bodei L, Ferrari M, Grana C, et al. Receptor-mediated radiotherapy with 90Y-DOTA-d-Phe1-Tyr3-octreotide. Eur J Nucl Med 2001;28(4):426–34.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, et al. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med Mol Imaging 2003;30(2):207–16.
Bodei L, Cremonesi M, Grana C, Bartolomei M, Baio SM, Bufi G, et al. Receptor radionuclide therapy with 177Lu-DOTATATE in neuroendocrine tumours. Eur J Nucl Med Mol Imaging 2006;33(Suppl 2):S214.
Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet 2007;370:591–603.
Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med 2006;354:2473–83.
Al-Said J, Brumback MA, Moghazi S, Baumgarten DA, O'Neill WC. Reduced renal function in patients with simple renal cysts. Kidney Int 2004;65(6):2303–8.
Yamazaki H, Oi H, Matsushita M, Kim T, Tanaka E, Inoue T, et al. Renal cortical retention on delayed CT and nephropathy following transcatheter arterial chemoembolisation. Br J Radiol 2001;74(884):695–700.
Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci 2007;334(2):115–24.
Savi A, Lecchi M, Albertini F, Chiesa C, Gilardi MC, Bombardieri E, et al. Evaluation of attenuation correction in planar In-111 biodistribution studies [abstract]. Eur J Nucl Med Mol Imaging 2004;31(Suppl):S229.
Cremonesi M, Ferrari M, Bodei L, Tosi G, Paganelli G. Dosimetry in peptide radionuclide receptor therapy: a review. J Nucl Med 2006;47(9):1467–75.
Cremonesi M, Ferrari M, Bodei L, Bartolomei M, Chinol M, Mei R, et al. Dosimetry in patients undergoing 177Lu-DOTATATE therapy with indications for 90Y-DOTATATE. Eur J Nucl Med Mol Imaging 2006;33(Suppl 1):S102. Abs 91.
Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates: MIRD pamphlet no16. J Nucl Med 1999;40(2):S37–S61.
Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 2005;46:1023–27.
Foster D, Barret P (editors). Developing and testing integrated multicompartment models to describe a single-input multiple-output study using the SAAM II software system. In: Proceedings of the Sixth International Radiopharmaceutical Dosimetry Symposium. Oak Ridge, Tennessee: Oak Ridge Institute for Science and Education;1998.
Dale R. Use of the linear-quadratic radiobiological model for quantifying kidney response in targeted radiotherapy. Cancer Biother Radiopharm 2004;19(3):363–70.
Thames HD, Ang KK, Stewart FA, van der Schueren E. Does incomplete repair explain the apparent failure of the basic LQ model to predict spinal cord and kidney responses to low doses per fraction? Int J Radiat Biol 1988;54(1):13–9.
Barone R, Borson-Chazot F, Valkema R, Walrand S, Chauvin F, Gogou L, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose–effect relationship. J Nucl Med 2005;46(Suppl 1):99S–106S.
Cybulla M, Weiner SM, Otte A. End-stage renal disease after treatment with 90Y-DOTATOC. Eur J Nucl Med 2001;28(10):1552–4.
Otte A, Herrmann R, Heppeler A, Behe M, Jermann E, Powell P, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med 1999;26(11):1439–47.
Valkema R, Pauwels SA, Kvols LK, Kwekkeboom DJ, Jamar F, de Jong M, et al. Long-term follow-up of renal function after peptide receptor radiation therapy with (90)Y-DOTA(0),Tyr(3)-octreotide and (177)Lu-DOTA(0), Tyr(3)-octreotate. J Nucl Med 2005;46(Suppl 1):83S–91S.
National Council on Radiation Protection and Measurements. Misadministration of radioactive material in medicine–scientific background. Bethesda, MD 1991:27
Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys 1995;31(5):1249–56.
Cremonesi M, Ferrari M, Zoboli S, Chinol M, Stabin MG, Orsi F, et al. Biokinetics and dosimetry in patients administered with 111In-DOTA-Tyr3-octreotide: implications for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med 1999;26:877–86.
Walrand S, Jamar F, Mathieu I, De Camps J, Lonneux M, Sibomana M, et al. Quantitation in PET using isotopes emitting prompt single gammas: application to yttrium-86. Eur J Nucl Med Mol Imaging 2003;30:354–61.
Dale R, Carabe-Fernandez A. The radiobiology of conventional radiotherapy and its application to radionuclide therapy. Cancer Biother Radiopharm 2005;20(1):47–51.
Acknowledgement
The authors thank William Russell-Edu for his kind assistance in revising the English of this manuscript.
Conflict of interest statement
None of the authors have any duality or conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00259-008-0914-y
Rights and permissions
About this article
Cite this article
Bodei, L., Cremonesi, M., Ferrari, M. et al. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. Eur J Nucl Med Mol Imaging 35, 1847–1856 (2008). https://doi.org/10.1007/s00259-008-0778-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-008-0778-1