Abstract
Purpose
Treatment with the radiolabelled somatostatin analogue 177Lu-octreotate results in tumour remission in 47% of patients with gastroenteropancreatic neuroendocrine tumours. Adding capecitabine to 177Lu-octreotate, as a radio-sensitiser, may enhance these anti-tumour effects. We now present the short-term toxicity profile of this novel combination.
Methods
Seven patients were treated with 7.4 GBq 177Lu-octreotate and capecitabine (1650 mg/m2 per day) for 2 weeks with an intended number of four cycles. Toxicity, and especially haematological and renal parameters, were monitored on a weekly basis for the first two cycles and 4 and 6 weeks after subsequent cycles.
Results
None of the patients had hand-foot syndrome. One patient had grade 1 stomatitis occurring after one of four cycles. Grade 3 or 4 leukopenia or neutropenia did not occur. One patient had grade 3 anaemia, but none had grade 4 anaemia. One patient had grade 2 thrombocytopenia after the fourth cycle, and one had grade 3 thrombocytopenia. Grade 4 thrombocytopenia did not occur. No significant changes in serum creatinine levels were observed. None of the patients had symptoms of cardiac ischaemia.
Conclusions
Treatment with the combination of 177Lu-octreotate and capecitabine was feasible and safe considering acute and subacute side effects. We therefore started a randomised, controlled clinical trial to compare this combination with 177Lu-octreotate as single agent with regard to anti-tumour effects and side effects.
Similar content being viewed by others
Introduction
Peptide receptor radionuclide therapy (PRRT) with radiolabelled somatostatin analogues can be very rewarding in patients with inoperable somatostatin receptor positive gastroenteropancreatic neuroendocrine tumours (GEP NETs). Treatment with the radiolabelled analogue [177Lu-DOTA0, Tyr3]octreotate (177Lu-octreotate) resulted in tumour remission in 47% of 125 patients, and the median time to progression had not been reached after 36 months of follow-up [1]. The most frequently occurring short-term side effects of this treatment were mild reversible alopecia in 64% of patients, nausea in 31%, vomiting in 14% and an increase in pain in tumour-involved regions in 12%. WHO haematological toxicity in 131 patients treated with 177Lu-octreotate was as follows: Hb grade 3 and 4 in 0.4 and 0.0%, leukopenia grade 3 and 4 in 1.3 and 0.0% and thrombocytopenia grade 3 and 4 in 1.5 and 0.2%.
Although the anti-tumour effects of therapy with 177Lu-octreotate are promising and serious side effects are rare, further studies are needed to find treatments that are more effective and still have acceptable side effects. One option for improvement is to use combinations of a radiolabelled somatostatin analogue with chemotherapeutic agents as radio-sensitiser. Candidate drugs for this purpose are 5-fluorouracil (5-FU) and capecitabine, which is an oral prodrug of 5-FU. For 5-FU, the additional effect to radiation therapy has been demonstrated: The combination of external beam radiation therapy with 5-FU resulted in better anti-tumour effects than external beam radiation therapy alone in patients with cancers of stomach, pancreas, large bowel, rectum, head and neck and oesophagus (mostly with cisplatin added as well). Moreover, chemoradiotherapy with 5-FU is also successfully used in anal and bladder cancers (see [2] for review). External beam radiation therapy with capecitabine was as effective as external beam radiation therapy with intravenous 5-FU in patients with locally advanced rectal carcinoma, but treatment with capecitabine is more convenient for patients, as it is an oral drug [3, 4]. Most common specific side effects of capecitabine are hand-foot syndrome and stomatitis. These side effects occur frequently when capecitabine is used in a dose of 2,500 mg/m2 per day in chemotherapy regimens, either as single agent or in combination with other chemotherapeutic agents. When using capecitabine as radio-sensitiser, lower doses (1,600-2,000 mg/m2 per day) are administered, and hand-foot syndrome and stomatitis are less frequent. Another known, but rather infrequent, side effect of capecitabine and related drugs is cardiac ischaemia. This can result in myocardial infarction, also in patients without a previous history of cardiac disease [5].
Most data on the combination of radiation therapy and 5-FU or capecitabine were derived from studies using external beam radiation therapy. Few data have been published about 5-FU or capecitabine in combination with radionuclide-derived radiation therapy with radiolabelled peptides or antibodies. Wong et al. [6] reported that treatment with 90Y-labelled antibodies and 5-FU was safe in patients with metastasised colorectal cancer. PRRT with [111In-DTPA0]octreotide in combination with 5-FU resulted in symptomatic improvement in 71% of patients with neuroendocrine tumours [7]. This is much more than 13 out of 38 patients (34%) with symptomatic improvement published by Valkema et al. [8] who had not used 5-FU during PRRT with [111In-DTPA0]octreotide. We were therefore interested in combining 177Lu-octreotate with capecitabine in treating patients with GEP NETs, as this might result in better treatment outcomes. A possible drawback could be an increase in side effects. We therefore started a pilot study to investigate this combination.
We here present data on the acute toxicity profile of 177Lu-octreotate in combination with capecitabine in seven patients. We monitored side effects including nausea, vomiting, chest pain, hand-foot syndrome and stomatitis, haematological side effects and acute side effects on kidneys and liver: this to evaluate if this new combination is safe and feasible in patients.
Materials and methods
Patients
Seven patients with metastasised GEP NETs were studied. All patients had measurable disease. All patients had tumour tissue uptake with [111In-DTPA0] octreotide scintigraphy (OctreoScan®) that was on average equal to or higher than uptake in normal hepatic tissue on planar images. Patients with known somatostatin receptor-negative lesions were excluded. Patients had not been treated with other radiolabelled somatostatin analogues before. Prerequisites for the first treatment were haemoglobin (Hb) ≥5.5 mmol/l, WBC ≥2 × 109/l, platelets ≥100 × 109/l, serum creatinine ≤150 μmol/l and 24-h urine creatinine clearance ≥50 ml/min, and Karnofsky performance score ≥60. Haematological criteria for further treatments were: Hb ≥5.0 mmol/l, WBC ≥2 × 109/l, platelets ≥75 × 109/l. All other criteria for re-treatment were identical. All patients gave written informed consent to participate in the study, which was approved by the local medical ethical committee.
Methods
[DOTA0,Tyr3]octreotate was obtained from Mallinckrodt (St Louis, MO, USA). 177LuCl3 was obtained from NRG (Petten, The Netherlands) and was distributed by IDB-Holland (Baarle-Nassau, The Netherlands). 177Lu-octreotate was locally prepared as described previously [9].
Granisetron 3 mg was injected intravenously 30 min before starting the infusion of 177Lu-octreotate. To reduce radiation dose to the kidneys, an infusion of amino acids (arginine 2.5% and lysine 2.5%) was also started 30 min before the administration of the radiopharmaceutical and lasted 4 h. Via a second pump system, the radiopharmaceutical was co-administered. Cycle doses were 7.4 GBq injected in 30 min. The interval between treatments was 6-10 weeks. Patients were treated up to an intended cumulative dose of 29.6 GBq.
Capecitabine (Xeloda®; Roche, Basel, Switzerland) 1,650 mg/m2 per day, divided over two doses was administered orally, starting on the day of treatment with 177Lu-octreotate and continuing for 14 days. Patients were instructed to report any signs of hand-foot syndrome, stomatitis or other side effects.
Routine haematology, liver and kidney function tests were performed after each therapy. This was performed every week after the first and second treatment cycle. After subsequent cycles, blood tests were performed at 4 and 6 weeks after the treatment. Figure 1 depicts the treatment schedule including blood tests.
Time schedule of treatment with 177Lu-octreotate and capecitabine and follow-up during treatment. Each regular treatment cycle consisted of an injection of 177Lu-octreotate (7.4 GBq) and of capecitabine (1650 mg/m2 per day) for 2 weeks. Blood tests were performed at 4 and 6 weeks after start of a treatment cycle. In this pilot study, blood tests were done every week only after the first and second cycles. The next treatment cycle was given 6 to 10 weeks later. The intended number of cycles was four
Results
Seven male patients were studied. All had metastasised GEP NETs (three non-functioning pancreatic NETs, four carcinoid tumours). Six had progressive disease, demonstrated within 12 months before start of treatment. In one patient, some growth occurred before start (interval between scans of 2 months), but this did not classify as progressive disease according to Southwest oncology group criteria. In this patient, treatment was started because of a large hepatic tumour load. Age at start ranged from 36 to 67 years (median 62 years). None of the patients had been treated with capecitabine or other chemotherapeutic agents before.
Twenty-six treatment cycles were given: Six patients received four cycles; in one patient, treatment was stopped after two cycles because of clinically progressive disease. Side effects within 24 h after administration were: nausea with vomiting, reported by one patient after one cycle. Two patients had an increase in diarrhoea (3 of 26 cycles). Increase in pain was not reported by any of the patients. Patients also reported subacute side effects in the weeks after the treatment: Five patients had nausea at home (7 of 26 cycles), two patients vomited (5 of 26 cycles). Two patients noticed an increase in pain in tumour-involved areas (2 of 26 cycles). Six patients experienced some fatigue after a treatment cycle (7 of 26 cycles). Mild alopecia was observed in four patients.
The patients were also asked to report any side effects that are more specific for capecitabine: None of the patients had signs or symptoms of hand-foot syndrome. One patient mentioned that after the second treatment, the oral mucosa was a bit more sensitive than usual, but no mucosal abnormalities were seen (grade 1 stomatitis). None of the other patients had complaints of stomatitis (Table 1). None of the patients had chest pains. One patient with a serotonin producing metastasised midgut carcinoid developed mild oedema of the lower extremities. This was due to severely progressive tricuspid valve insufficiency proven by a cardiac ultrasound. Another patient developed oedema of the legs caused by compression of the inferior vena cava. None of the other patients had symptoms of heart failure.
Haematological toxicity is summarised in Table 2. Grade 3 or 4 leukopenia or neutropenia did not occur. One patient had grade 3 anaemia (Hb 4.9 mmol/l) after the second cycle, which resolved to grade 2 spontaneously 1 week later. In this patient, treatment was stopped after two cycles because of clear clinical progression of disease. Grade 4 anaemia did not occur. One patient had grade 2 thrombocytopenia (72 × 109/l) after the fourth treatment. In one patient, platelet count dropped to 36 × 109/l (grade 3 toxicity) after the third cycle. After recovery of platelet count to 93 × 109/l, therapy was resumed with half a dose of 177Lu-octreotate (3.7 GBq) and the normal dose of capecitabine. Subsequently, platelet count again dropped to 37 × 109/l. Treatment with 177Lu-octreotate was then stopped (cumulative dose 25.6 GBq) because the patient was deteriorating clinically and progression of disease was documented on CT scan. Grade 4 thrombocytopenia did not occur.
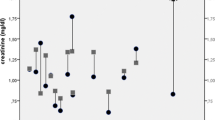
The course of haematological parameters and serum creatinine levels are shown in Fig. 2. Platelet counts at 4 weeks after treatment were consistently lower than baseline counts or counts at 6 weeks after the same treatment. No changes were noted in serum creatinine levels (no grade 2 or higher toxicity). No changes in liver function tests occurred that could be attributed to the treatment. Liver function tests deteriorated in the patient with progressive disease despite two treatment cycles.
Course of haematological parameters and serum creatinine levels in seven patients during and after treatments with 177Lu-octreotate and capecitabine (mean ± standard error of mean). Time axis: 0 represent baseline values, x·y: x represents treatment cycle, y represents weeks after treatment. Data after treatments 3 and 4 are based on six patients
Although not the subject of this study, it is reassuring to see that the treatment with the combination of 177Lu-octreotate and capecitabine resulted in tumour size reduction in the first patient we treated (Fig. 3).
The first patient treated with 177Lu-octreotate and capecitabine was a 36-year-old man with a metastasised pancreatic neuroendocrine tumour. Images in the left panel present the tumours at baseline; the right panel presents the situation 3 months after the fourth cycle. The patient had a partial remission
Discussion
PRRT with 177Lu-octreotate as single agent is effective in patients with somatostatin receptor positive gastroenteropancreatic neuroendocrine tumours. However, strategies to increase the efficacy of such treatment should be investigated. One possible way to improve these effects is combining 177Lu-octreotate with chemotherapeutic agents as radio-sensitiser. Capecitabine is often used as radio-sensitiser with external beam radiation therapy. It has attractive features for combining with radiation therapy: Capecitabine is an oral prodrug of 5-FU and has to be converted to its active form after three enzymatic converting steps. The third step is by the enzyme thymidine phosphorylase (TP). Several types of malignant cells have high expression of TP, and this can result in higher concentrations of the active form (i.e. 5-FU) in tumour cells compared to non-malignant cells [10]. Moreover, TP expression is induced by radiation [11], which can again result in higher concentrations of 5-FU in irradiated cells. These features are also attractive for combining capecitabine with radionuclide-derived radiation therapy, like PRRT.
To our knowledge, no studies have so far been published that describe the combination of capecitabine with a somatostatin analogue labelled with a beta-emitting isotope, like 177Lu-octreotate, with regard to side effects. Based on the findings from a pilot study to evaluate the safeness and feasibility of this combination, we intended to make a decision to start or reject a randomised clinical trial comparing 177Lu-octreotate as single agent with 177Lu-octreotate in combination with capecitabine.
Haematological toxicity was infrequent. One patient had grade 2 thrombocytopenia after the fourth cycle. In one patient, WHO grade 3 thrombocytopenia occurred after the third and fourth cycles. In another patient, haemoglobin was 4.9 mmol/l on one occasion (WHO grade 3 anaemia) after the second cycle, which improved within 1 week to grade 2 anaemia.
No acute renal toxicity was observed in these patients based on measured serum creatinine levels. Of course, some subtle side effects on glomerular filtration rate or tubular function, which may only be demonstrated with more sensitive methods, like 99mTc-DTPA or 99mTc-MAG3, cannot be ruled out. However, based on serum creatinine levels alone, we may conclude that there was no clinically relevant acute renal toxicity.
None of the patients had hand-foot syndrome, and one patient had a more sensitive oral mucosa, but grade 2 or more stomatitis was not noted. The low frequency of these side effects of capecitabine in our group can be explained by the relatively low dose (approximately 825 mg/m2 bid) used in this and other radio-sensitising studies. This is an important characteristic, as ideally, we do not want to provoke side effects that have a serious impact on quality of life in these patients who usually have a life expectancy of several years. Furthermore, nausea, vomiting and hair loss were observed, but percentages in the group treated with the combination are similar to those after treatment with 177Lu-octreotate alone. None of the patients had symptoms of cardiac ischaemia or heart failure that could be attributed to capecitabine.
Of note is that so far, only acute and subacute side effects could be registered. No data are known yet about long-term side effects. The patients treated with the combination of 177Lu-octreotate and capecitabine will therefore also be closely monitored in the future to reveal potential late toxic effects, e.g. on kidney function and bone marrow. The patients will undergo blood tests every 6 months and will also collect urine for 24 h to determine creatinine clearance and proteinuria.
Based on these findings, we conclude that the combination of 177Lu-octreotate with capecitabine is safe and feasible in patients with GEP NETs with regard to short-term side effects. We recently started a randomised controlled clinical trial comparing treatment with 177Lu-octreotate alone to treatment with 177Lu-octreotate and capecitabine. Ultimately, the results of that study will provide final data about differences in anti-tumour effects and toxicity profiles between these treatments, also on the longer term.
References
Kwekkeboom DJ, Teunissen JJ, Bakker WH, Kooij PP, de Herder WW, Feelders RA, et al. Treatment with the radiolabeled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate in patients with gastro-entero-pancreatic (GEP) tumors. J Clin Oncol 2005;23:2754-62.
Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 2004;22:2214-32.
Das P, Lin EH, Bhatia S, Skibber JM, Rodriguez-Bigas MA, Feig BW, et al. Preoperative chemoradiotherapy with capecitabine versus protracted infusion 5-fluorouracil for rectal cancer: a matched pair analysis. Int J Radiation Oncology Biol Phys 2006;66:1378-83.
Kim DY, Jung KH, Kim TH, Kim DW, Chang HJ, Jeong JY, et al. Comparison of 5-fluorouracil/leucovorin and capecitabine in preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Radiation Oncology Biol Phys 2007;67:378-84.
Kosmas C, Kallistratos MS, Kopterides P, Syrios J, Skopelitis H, Mylonakis N, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 2008;134:75-82.
Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, et al. A phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res 2003;9:5842-52.
Kong G, Lau E, Ramdave S, Hicks RJ. High-dose In-111 octreotide therapy in combination with radiosensitizing 5-FU chemotherapy for treatment of SSR-expressing neuroendocrine tumors. [Abstract] J Nucl Med 2005;46(Suppl 2):151P.
Valkema R, de Jong M, Bakker WH, Breeman WA, Kooij PP, Lugtenburg PJ, et al. Phase I study of peptide receptor radionuclide therapy with [111In-DTPA0]Octreotide: the Rotterdam experience. Semin Nucl Med 2002;32:110-22.
Kwekkeboom DJ, Bakker WH, Kooij PP, Konijnenberg MW, Srinivasan A, Erion JL, et al. [177Lu-DOTA0Tyr3]octreotate: comparison with [111In-DTPA0]octreotide in patients. Eur J Nucl Med 2001;28:1319-25.
Miwa M, Ura M, Nishida M, Sawada N, Ishikawa T, Mori K, et al. Design of a novel oral fluoropyrimidine carbamate, capecitabine, which generates 5-fluorouracil selectively in tumours by enzymes concentrated in human liver and cancer tissue. Eur J Cancer 1998;34:1274-81.
Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res 1999;5:2948-53.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Essen, M., Krenning, E.P., Kam, B.L. et al. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging 35, 743–748 (2008). https://doi.org/10.1007/s00259-007-0688-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0688-7