Abstract
Purpose
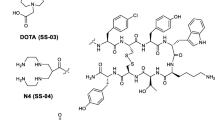
Gallium-68 is a metallic positron emitter with a half-life of 68 min that is ideal for the in vivo use of small molecules, such as [68Ga-DOTA,Tyr3]octreotide, in the diagnostic imaging of somatostatin receptor-positive tumours. In preclinical studies it has shown a striking superiority over its 111In-labelled congener. The purpose of this study was to evaluate whether third-generation somatostatin-based, radiogallium-labelled peptides show the same superiority.
Methods
Peptides were synthesised on solid phase. The receptor affinity was determined by in vitro receptor autoradiography. The internalisation rate was studied in AR4-2J and hsst-HEK-transfected cell lines. The pharmacokinetics was studied in a rat xenograft tumour model, AR4-2J.
Results
All peptides showed high affinities on hsst2, with the highest affinity for the GaIII-complexed peptides. On hsst3 the situation was reversed, with a trend towards lower affinity of the GaIII peptides. A significantly increased internalisation rate was found in sst2-expressing cells for all 67Ga-labelled peptides. Internalisation into HEK-sst3 was usually faster for the 111In-labelled peptides. No internalisation was found into sst5. Biodistribution studies employing [67Ga-DOTA,1-Nal3]octreotide in comparison to [111In-DOTA,1-Nal3]octreotide and [67Ga-DOTA,Tyr3]octreotide showed a significantly higher and receptor-mediated uptake of the two 67Ga-labelled peptides in the tumour and somatostatin receptor-positive tissues. A patient study illustrated the potential advantage of a broad receptor subtype profile radiopeptide over a high-affinity sst2-selective radiopeptide.
Conclusion
This study demonstrates that 67/68Ga-DOTA-octapeptides show distinctly better preclinical, pharmacological performances than the 111In-labelled peptides, especially on sst2-expressing cells and the corresponding animal models. They may be excellent candidates for further development for clinical studies.




Similar content being viewed by others
References
Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003;24:389–427.
Maina T, Nock B, Nikolopoulou A, Sotiriou P, Loudos G, Maintas D, et al. [99mTc]Demotate, a new 99mTc-based [Tyr3]octreotate analogue for the detection of somatostatin receptor-positive tumours: synthesis and preclinical results. Eur J Nucl Med Mol Imaging 2002;29:742–53.
Decristoforo C, Mather SJ, Cholewinski W, Donnemiller E, Riccabona G, Moncayo R. 99mTc-EDDA/HYNIC-TOC: a new 99mTc-labelled radiopharmaceutical for imaging somatostatin receptor-positive tumours; first clinical results and intra-patient comparison with 111In-labelled octreotide derivatives. Eur J Nucl Med 2000;27:1318–25.
Storch D, Behe M, Walter MA, Chen J, Powell P, Mikolajczak R, et al. Evaluation of [99mTc/EDDA/HYNIC0]octreotide derivatives compared with [111In-DOTA0,Tyr3, Thr8]octreotide and [111In-DTPA0]octreotide: does tumor or pancreas uptake correlate with the rate of internalization? J Nucl Med 2005;46:1561–9.
de Jong M, Bakker WH, Krenning EP, Breeman WA, van der Pluijm ME, Bernard BF, et al. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0,d-Phe1,Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997;24:368–71.
Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WA, Kooij PP, Oei HY, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716–31.
Smith-Jones PM, Stolz B, Bruns C, Albert R, Reist HW, Fridrich R, et al. Gallium-67/gallium-68-[DFO]-octreotide—a potential radiopharmaceutical for PET imaging of somatostatin receptor-positive tumors: synthesis and radiolabeling in vitro and preliminary in vivo studies. J Nucl Med 1994;35:317–25.
Henriksen G, Schottelius M, Poethko T, Hauser A, Wolf I, Schwaiger M, et al. Proof of principle for the use of 11C-labelled peptides in tumour diagnosis with PET. Eur J Nucl Med Mol Imaging 2004;31:1653–7.
Wester H-J, Schottelius M, Scheidhauer K, Meisetschläger G, Herz M, Rau F, et al. PET imaging of somatostatin receptors: design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging 2002;30:117–22.
Sprague JE, Peng Y, Sun X, Weisman GR, Wong EH, Achilefu S, et al. Preparation and biological evaluation of copper-64-labeled Tyr3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res 2004;10:8674–82.
Waldherr C, Pless M, Maecke H, Schumacher T, Crazzolara A, Nitzsche E, et al. Tumor response and clincical benefit in neuroendocrine tumors after 7.4 GBq 90Y-DOTATOC. J Nucl Med 2002;43:610–6.
Otte A, Mueller-Brand J, Dellas S, Nitzsche E, Herrmann R, Maecke H. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet 1998;351:417–8.
Kwekkeboom DJ, Mueller-Brand J, Paganelli G, Anthony LB, Pauwels S, Kvols LK, et al. Overview of results of peptide receptor radionuclide therapy with 3 radiolabeled somatostatin analogs. J Nucl Med 2005;46:62S–66S.
Bodei L, Cremonesi M, Zoboli S, Grana C, Bartolomei M, Rocca P, et al. Receptor-mediated radionuclide therapy with 90Y-DOTATOC in association with amino acid infusion: a phase I study. Eur J Nucl Med 2003;30:207–16.
de Jong M, Breeman WA, Bernard BF, Bakker WH, Schaar M, van Gameren A, et al. [177Lu-DOTA0,Tyr3]octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer 2001;92:628–33.
Norenberg JP, Krenning BJ, Konings IR, Kusewitt DF, Nayak TK, Anderson TL, et al. 213Bi-[DOTA0, Tyr3]octreotide peptide receptor radionuclide therapy of pancreatic tumors in a preclinical animal model. Clin Cancer Res 2006;12:897–903.
Jensen RT. Carcinoid and pancreatic endocrine tumors: recent advances in molecular pathogenesis, localization, and treatment. Curr Opin Oncol 2000;12:368–77.
Maecke HR, Hofmann M, Haberkorn U. 68Ga-labeled peptides in tumor imaging. J Nucl Med 2005;46:172S–8S.
Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjug Chem 2002;13:530–41.
Heppeler A, Froidevaux S, Mäcke HR, Jermann E, Béhé M, Powell P, et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour-targeting properties and potential for receptor-mediated internal radiotherapy. Chemistry A European Journal 1999;5:1016–23.
Froidevaux S, Eberle AN, Christe M, Sumanovski L, Heppeler A, Schmitt JS, et al. Neuroendocrine tumor targeting: study of novel gallium-labeled somatostatin radiopeptides in a rat pancreatic tumor model. Int J Cancer 2002;98:930–7.
Hofmann M, Maecke H, Börner A, Weckesser E, Schöffski P, Oei M, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med 2001;28:1751–7.
Kowalski J, Henze M, Schuhmacher J, Maecke HR, Hofmann M, Haberkorn U. Evaluation of positron emission tomography imaging using [68Ga]-DOTA-D Phe1-Tyr3-octreotide in comparison to [111In]-DTPAOC SPECT. First results in patients with neuroendocrine tumors. Mol Imaging Biol 2003;5:42–8.
Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, et al. Characterization of 68Ga-DOTA-D-Phe1-Tyr3-octreotide kinetics in patients with meningiomas. J Nucl Med 2005;46:763–9.
Henze M, Schuhmacher J, Dimitrakopoulou-Strauss A, Strauss LG, Maecke HR, Eisenhut M, et al. Exceptional increase in somatostatin receptor expression in pancreatic neuroendocrine tumour, visualised with 68Ga-DOTATOC PET. Eur J Nucl Med Mol Imaging 2004;31:466.
Henze M, Schumacher T, Hipp P, Kowalski J, Becker D, Doll J, et al. PET imaging of somatostatin receptors using [68Ga]DOTA-D-Phe1-Tyr3-octreotide: first results in patients with meningiomas. J Nucl Med 2001;42:1053–6.
Dimitrakopoulou-Strauss A, Georgoulias V, Eisenhut M, Herth F, Koukouraki S, Macke HR, et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using 68Ga-DOTATOC PET and comparison with 18F-FDG PET. Eur J Nucl Med Mol Imaging 2006;33:823–30.
Baum R, Niesen A, Leonhardi J, Wortmann R, Mueller D, Roesch F. Receptor PET/CT imaging of neuroendocrine tumours using the Ga-68 labelled, high affinity somatostatin analogue DOTA-1-Nal3 octreotide (DOTA-NOC): clinical results in 327 patients. Eur J Nucl Med Mol Imaging 2005;32 Suppl 1:S54–5.
Roesch F, Zhernosekov K, Filosofov D, Jahn M, Jennewein M, Baum R, et al. Processing of Ge-68/Ga-68 generator eluates for labeling of biomolecules via bifunctional chelators. J Nucl Med 2006;47 Suppl 1:162P.
Baum R, Schmücking M, Wortmann R, Müller M, Zhernosekov K, Rösch F. Receptor PET/CT using the Ga-68 labelled somatostatin analog DOTA-1-Nal3-octreotide (DOTA-NOC): clinical experience in 140 patients. Nuklearmedizin 2005;44:A57.
Rufini V, Calcagni ML, Baum RP. Imaging of neuroendocrine tumors. Semin Nucl Med 2006;36:228–47.
Win Z, Rahman L, Murrell J, Todd J, Al-Nahhas A. The possible role of 68Ga-DOTATATE PET in malignant abdominal paraganglioma. Eur J Nucl Med Mol Imaging 2006;33:506.
Wild D, Schmitt JS, Ginj M, Maecke HR, Bernard BF, Krenning E, et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging 2003;30:1338–47.
Ginj M, Chen J, Walter MA, Eltschinger V, Reubi JC, Maecke HR. Preclinical evaluation of new and highly potent analogues of octreotide for predictive imaging and targeted radiotherapy. Clin Cancer Res 2005;11:1136–45.
Reubi JC, Schar JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000;27:273–82.
Tulipano G, Stumm R, Pfeiffer M, Kreienkamp HJ, Hollt V, Schulz S. Differential β-arrestin trafficking and endosomal sorting of somatostatin receptor subtypes. J Biol Chem 2004;279:21374–82.
Smith-Jones PM, Solit DB, Akhurst T, Afroze F, Rosen N, Larson SM. Imaging the pharmacodynamics of HER2 degradation in response to Hsp90 inhibitors. Nat Biotechnol 2004;22:701–6.
Smith-Jones PM, Solit D, Afroze F, Rosen N, Larson SM. Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med 2006;47:793–6.
Wild D, Maecke HR, Waser B, Reubi JC, Ginj M, Rasch H, et al. 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging 2004;32:724.
Decristoforo C, von Guggenberg E, Haubner R, Rupprich M, Schwarz S, Virgolini I. Radiolabeling of DOTA-derivatised peptides with 68Ga via a direct approach—optimization and routine clinical application. Nuklearmedizin 2005;44:A191–2.
Hofmann M, Oei M, Boerner AR, Maecke H, Geworski L, Knapp WH, et al. Comparison of Ga-68-DOTATOC and Ga-68-DOTANOC for radiopeptide PET. Nuklearmedizin 2005;44:A58.
Cescato R, Schulz S, Waser B, Eltschinger V, Rivier JE, Wester HJ, et al. Internalization of sst2, sst3, and sst5 receptors: effects of somatostatin agonists and antagonists. J Nucl Med 2006;47:502–11.
Smith SV. Molecular imaging with copper-64. J Inorg Biochem 2004;98:1874–901.
Rossin R, Pan D, Qi K, Turner JL, Sun X, Wooley KL, et al. 64Cu-labeled folate-conjugated shell cross-linked nanoparticles for tumor imaging and radiotherapy: synthesis, radiolabeling, and biologic evaluation. J Nucl Med 2005;46:1210–8.
Boswell CA, Sun X, Niu W, Weisman GR, Wong EH, Rheingold AL, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem 2004;47:1465–74.
Acknowledgements
P. Antunes acknowledges the PhD Fellowship of the Fundação para a Ciência e Tecnologia (Ref. SFRH/BD/3136/2000). In addition, P. Antunes, M. Ginj, M. Walter and H. Maecke acknowledge the support from the Swiss National Science Foundation project No. 3100A0-100390, BBW project No C00.0091, and the network of excellence, European Molecular Imaging Laboratories (EMIL). The support provided by Novartis Pharma in respect of ESI-MS analysis is gratefully acknowledged. We thank Dr. S. Schulz for the sst3-transfected human embryonic kidney 293 cells. The authors thank K. Hinni and S. Tschumi for biological technical assistance. This work was performed within the COST B12 Action.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Antunes, P., Ginj, M., Zhang, H. et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals?. Eur J Nucl Med Mol Imaging 34, 982–993 (2007). https://doi.org/10.1007/s00259-006-0317-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0317-x