Abstract
Purpose
[7-methyl-11C]-(E)-8-(3,4,5-trimethoxystyryl)-1,3,7-trimethylxanthine ([11C]TMSX) is a positron-emitting adenosine A2A receptor (A2AR) antagonist for visualisation of A2AR distribution by positron emission tomography (PET). The aims of this paper were to use a kinetic model to analyse the behaviour of [11C]TMSX in the brain and to examine the applicability of the Logan plot. We also studied the applicability of a simplified Logan plot by omitting metabolite correction and arterial blood sampling.
Methods
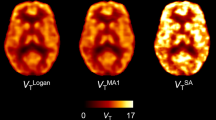
The centrum semiovale was used as a reference region on the basis of a post-mortem study showing that it has a negligibly low density of A2ARs. Compartmental analysis was performed in five normal subjects. Parametric images of A2AR binding potential (BP) were also generated using a Logan plot with or without metabolite correction and with or without arterial blood sampling. To omit arterial blood sampling, we applied a method to extract the plasma-related information using independent component analysis (EPICA).
Results
The estimated K 1/k 2 was confirmed to be common in the centrum semiovale and main cortices. The three-compartment model was well fitted to the other regions using the fixed value of K 1/k 2 estimated from the centrum semiovale. The estimated BPs using the Logan plot matched those derived from compartment analysis. Without the metabolite correction, the estimate of BP underestimated the true value by 5%. The estimated BPs agreed regardless of arterial blood sampling.
Conclusion
A three-compartment model with a reference region, the centrum semiovale, describes the kinetic behaviour of [11C]TMSX PET images. A2ARs in the human brain can be visualised as a BP image using [11C]TMSX PET without arterial blood sampling.










Similar content being viewed by others
References
Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 2001;53:527–52.
Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse 1997;27:322–35.
Ishiwata K, Noguchi J, Wakabayashi S, Shimada J, Ogi N, Nariai T, et al. 11C-labeled KF18446: a potential central nervous system adenosine A2a receptor ligand. J Nucl Med 2000;41:345–54.
Ishiwata K, Wang WF, Kimura Y, Kawamura K, Ishii K. Preclinical studies on [11C]TMSX for mapping adenosine A2A receptors by positron emission tomography. Ann Nucl Med 2003;17:205–11.
Ishiwata K, Mishina M, Kimura Y, Oda K, Sasaki T, Ishii K. First visualization of adenosine A2A receptors in the human brain by positron emission tomography with [11C]TMSX. Synapse 2005;55:133–6.
Ishiwata K, Kawamura K, Kimura Y, Oda K, Ishii K. Potential of an adenosine A2A receptor antagonist [11C]TMSX for myocardial imaging by positron emission tomography: a first human study. Ann Nucl Med 2003;17:457–62.
Ishiwata K, Mizuno M, Kimura Y, Kawamura K, Oda K, Sasaki T, et al. Potential of [11C]TMSX for the evaluation of adenosine A2A receptors in the skeletal muscle by positron emission tomography. Nucl Med Biol 2004;31:949–56.
Mizuno M, Kimura Y, Tokizawa K, Ishii K, Oda K, Sasaki T, et al. Greater adenosine A2A receptor densities in cardiac and skeletal muscle in endurance trained men: a [11C]TMSX PET study. Nucl Med Biol 2005;32:831–6.
Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 1990;10:740–7.
Logan J. Graphical analysis of PET data applied to reversible and irreversible tracers. Nucl Med Biol 2000;27:661–70.
Watabe H, Channing MA, Der MG, Adams R, Jagoda E, Herscovitch P, et al. Kinetic analysis of the 5-HT2A ligand [11C]MDL 100,907. J Cereb Blood Flow Metab 2000;20:899–909.
Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage 1996;4:153–8.
Kimura Y, Ishii K, Fukumitsu N, Oda K, Sasaki T, Kawamura K, et al. Quantitative analysis of adenosine A1 receptors in human brain using positron emission tomography and [1-methyl-11C]8-dicyclopropylmethl-1-methyl-3-propylxanthine. Nucl Med Biol 2004;31:975–81.
Coleman T, Branch MA. Nonlinear least-square. Optimization toolbox user's guide. Natick, MA: MathWorks Inc.; 1999; p. 3–11.
William HP, Saul AT, William TV, Brian PF. 15.2 fitting data to a straight line. Numerical recipes in C: the art of scientific computing. 2nd ed. New York: Cambridge University Press; 1992; p. 661–6.
Naganawa M, Kimura Y, Ishii K, Oda K, Ishiwata K, Matani A. Extraction of a plasma time-activity curve from dynamic brain PET images based on independent component analysis. IEEE Trans Biomed Eng 2005;51:201–10.
Naganawa M, Kimura Y, Nariai T, Ishii K, Oda K, Manabe Y, et al. Omission of serial arterial blood sampling in neuroreceptor imaging with independent component analysis. NeuroImage 2005;26:885–90.
Comon P. Independent component analysis, a new concept? Signal Process 1994;36:287–314.
Hyvärinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw 1999;10:626–34.
Naganawa M, Matani A, Kimura Y. Extraction of vessel-related information from PET images without continuous blood sampling using modified independent component analysis. Proceedings of the 23rd Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2001:2744–7.
Meyer JH, Ichise M. Modeling of receptor ligand data in PET and SPECT imaging: a review of major approaches. J Neuroimaging 2001;11:30–9.
Koeppe RA, Holthoff VA, Frey KA, Kilbourn MR, Kuhl DE. Compartmental analysis of [11C]flumazenil kinetics for the estimation of ligand transport rate and receptor distribution using positron emission tomography. J Cereb Blood Flow Metab 1991;11:735–44.
Kropholler MA, Boellard R, Schuitemaker A, Berckel BNM, Luurtsema G, Windhorst AD, et al. Development of a tracer kinetic plasma input model for (R)-[11C]PK11195 brain studies. J Cereb Blood Flow Metab 2005;25:842–51.
Cunha RA, Johansson B, Constantino MD, Sebastiao AM, Fredholm BB. Evidence for high-affinity binding sites for the adenosine A2A receptor agonist [3H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A2A receptors. Naunyn Schmiedebergs Arch Pharmacol 1996;353:261–71.
Lindstrom K, Ongini E, Fredholm BB. The selective adenosine A2A receptor antagonist SCH 58261 discriminates between two different binding sites for [3H]-CGS 21680 in the rat brain. Naunyn Schmiedebergs Arch Pharmacol 1996;354:539–41.
Ishiwata K, Ogi N, Shimada J, Nonaka H, Tanaka A, Suzuki F, et al. Further characterization of a CNS adenosine A2a receptor ligand [11C]KF18446 with in vitro autoradiography and in vivo tissue uptake. Ann Nucl Med 2000;14:81–9.
Fredholm BB, Svenningsson P. Adenosine–dopamine interactions: development of a concept and some comments on therapeutic possibilities. Neurology 2003;61:S5–S9.
Bara-Jimenez W, Sherzai A, Dimitrova T, Favit A, Bibbiani F, Gillespie M, et al. Adenosine A2A receptor antagonist treatment of Parkinson’s disease. Neurology 2003;61:293–6.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research, No. 16390348 in 2004-2006, No. 18591373 in 2006-2007 and No. 18-6916 in 2006-2008 from the Japan Society for the Promotion of Science, and a Grant-in-Aid for the 21st Century COE from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naganawa, M., Kimura, Y., Mishina, M. et al. Quantification of adenosine A2A receptors in the human brain using [11C]TMSX and positron emission tomography. Eur J Nucl Med Mol Imaging 34, 679–687 (2007). https://doi.org/10.1007/s00259-006-0294-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0294-0