Abstract
Purpose
We aimed to determine the composition of radioactivity in rat brain after intravenous administration of the dopamine transporter radioligand, [11C]PE2I.
Methods
PET time-activity curves (TACs) and regional brain distribution ex vivo were measured using no-carrier-added [11C]PE2I. Carrier-added [11C]PE2I was administered to identify metabolites with high-performance liquid radiochromatography (RC) or RC with mass spectrometry (LC-MS and MS-MS). The stability of [11C]PE2I was assessed in rat brain homogenates.
Results
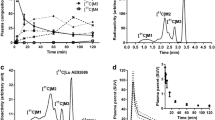
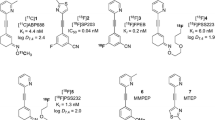
After peak brain uptake of no-carrier-added [11C]PE2I, there was differential washout rate from striata and cerebellum. Thirty minutes after injection, [11C]PE2I represented 10.9 ± 2.9% of the radioactivity in plasma, 67.1 ± 11.0% in cerebellum, and 92.5 ± 3.2% in striata, and was accompanied by two less lipophilic radiometabolites. [11C]PE2I was stable in rat brain homogenate for at least 1 h at 37°C. LC-MS identified hydroxylated PE2I (1) (m/z 442) and carboxyl-desmethyl-PE2I (2) (m/z 456) in brain. MS-MS of 1 gave an m/z 442→424 transition due to H2O elimination, so verifying the presence of a benzyl alcohol group. Metabolite 2 was the benzoic acid derivative. Ratios of ex vivo measurements of [11C]PE2I, [11C]1, and [11C]2 in striata to their cognates in cerebellum were 6.1 ± 3.4, 3.7 ± 2.2 and 1.33 ± 0.38, respectively, showing binding selectivity of metabolite [11C]1 to striata.
Conclusion
Radiometabolites [11C]1 and [11C]2 were characterized as the 4-hydroxymethyl and 4-carboxyl analogs of [11C]PE2I, respectively. The presence of the pharmacologically active [11C]1 and the inactive [11C]2 is a serious impediment to successful biomathematical analysis.








Similar content being viewed by others
References
Nurmi E, Ruottinen HM, Kaasinen V, Bergman J, Haaparanta M, Solin O, et al. Progression in Parkinson’s disease: a positron emission tomography study with a dopamine transporter ligand [18F]CFT. Ann Neurol 2000;47:804–8.
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, et al. Decreased SPECT [123I]ß-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 1995;38:589–98.
Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson disease. Proc Natl Acad Sci U S A 1993;90:11965–9.
Meyer JH, Krüger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, et al. Lower dopamine transporter binding potential in striatum during depression. NeuroReport 2001;12:4121–5.
Laakso A, Bergman J, Haaparanta M, Vilkman H, Solin O, Syvälahti E, et al. Decreased striatal dopamine transporter binding in vivo in chronic schizophrenia. Schizophr Res 2001;52:115–20.
Malison RT, McDougle CJ, van Dyck CH, Scahill L, Baldwin RM, Seibyl JP, et al. [123I]beta-CIT SPECT imaging of striatal dopamine transporter binding in Tourette’s disorder. Am J Psychiatry 1995;152:1359–61.
Emond P, Garreau L, Chalon S, Boazi M, Caillet M, Bricard J, et al. Synthesis and ligand binding of nortropane derivatives: N-substituted 2β-crabomethoxy-3β-(4’-iodophenyl)nortropane and N-(3-iodoprop-(2E)-enyl)-2β-carbomethoxy-3β-(3’,4’-disubstituted phenyl)nortropane. New high-affinity and selective compounds for the dopamine transporter. J Med Chem 1997;40:1366–72.
Guilloteau D, Emond P, Baulieu J-L, Garreau L, Frangin Y, Pourcelot L, et al. Exploration of the dopamine transporter: in vitro and in vivo characterization of a high-affinity and high-specificity iodinated tropane derivative (E)-N-(3-iodoprop-2-enyl)-2β-carbomethoxy-3β-(4’-methylphenyl)nortropane (PE2I). Nucl Med Biol 1998;25:331–7.
Goodman MM, Keil R, Shoup TM, Eshima D, Eshima L, Kilts C, et al. Fluorine-18-FPCT: a PET radiotracer for imaging dopamine transporters. J Nucl Med 1997;38:119–26.
Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, et al. [18F]-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl Med Biol 2000;27:1–12.
Goodman MM, Kung M-P, Kabalka GW, Kung HF, Switzer R. Synthesis and characterization of radioiodinated N-(3-iodopropen-1-yl)-2β-carbomethoxy-3β-(4-chlorophenyl)tropanes: potential dopamine reuptake site imaging agents. J Med Chem 1994;37:1535–42.
Xing D, Chen P, Keil R, Kilts CD, Shi B, Camp VM, et al. Synthesis, biodistribution, and primate imaging of fluorine-18 labeled 2β-carbo-1’-fluoro-2-propoxy-3β-(4-chlorophenyl)tropanes. Ligands for the imaging of dopamine transporters by positron emission tomography. J Med Chem 2000;43:639–48.
Neumeyer JL, Wang S, Gao Y, Milius RA, Kula NS, Campbell A, et al. N-ω-Fluoroalkyl analogs of (1R)-2β-carbomethoxy-3β-(4-iodophenyl)tropane (β-CIT): radiotracers for positron emission tomography and single photon emission computed tomography imaging of dopamine transporters. J Med Chem 1994;37:1558–61.
Davis MR, Votaw JR, Bremner JD, Byas-Smith MG, Faber TL, Voll RJ, et al. Initial human PET imaging studies with the dopamine transporter ligand 18F-FECNT. J Nucl Med 2003;44:855–61.
Lu J-Q, Rodrigues-Gomez JA, Velasco I, Zoghbi SS, Liow J-S, Musachio JL, et al. PET imaging of DAT with [18F]FECNT in naive and Parkinsonian rats following embryonic stem cell transplantation [abstract]. NeuroImage 2004;22:T27–T28.
Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow J-S, et al. PET imaging of the dopamine transporter with 18F-2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)nortropane: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med 2006;47:520–7.
Kuikka JK, Baulieu JL, Hiltunen J, Halldin C, Bergström K, Farde L, et al. Pharmacokinetics and dosimetry of iodine-123 labelled PE2I in humans, a radioligand for dopamine transporter imaging. Eur J Nucl Med 1998;25:531–4.
Halldin C, Erixon-Lindroth N, Pauli S, Chou Y-H, Okubo Y, Karlsson P, et al. [11C]PE2I: a highly selective radioligand for PET examination of the dopamine transporter in monkey and human brain. Eur J Nucl Med Mol Imaging 2003;30:1220–30.
Jucaite A, Odano I, Olsson H, Pauli S, Halldin C, Farde L. Quantitative analyses of regional [11C]PE2I binding to the dopamine transporter in the human brain: a PET study. Eur J Nucl Med Mol Imaging 2006;33:657–68.
Jucaite A, Fernell E, Halldin C, Forssberg H, Farde L. Reduced midbrain dopamine transporter binding in male adolescents with attention-deficit/hyperactivity disorder: association between striatal dopamine markers and motor hyperactivity. Biol Psychiatry 2005;57:229–38.
Zoghbi SS, Shetty UH, Ichise M, Hong J, Musachio JL, Seneca N, et al. The dopamine transporter probe [11C]PE2I shows active and inactive radioactive metabolites in rat brain [abstract]. J Label Compds Radiopharm 2005;48 Suppl 1:S98.
Hawkins DR, editor. Biotransformations: a survey of the biotransformations of drugs and chemicals in animals. Cambridge: The Royal Society of Chemistry; 1992.
Jenner P, Testa B, editors. Concepts in drug metabolism. New York: Marcel Dekker, Inc.; 1980.
Woolf TF, editor. Handbook of drug metabolism. New York: Marcel Dekker, Inc.; 1999.
Guengerich FP. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol 2001;14:611–50.
Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, et al. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996.
Seidel J, Vaquero JJ, Green MV. Resolution uniformity and sensitivity of the NIH ATLAS small animal PET scanner: comparison to simulated LSO scanners without depth-of-interaction capability. IEEE Trans Nucl Sci 2003;50:1347–50.
Johnson CA, Seidel J, Vaquero JJ, Pascau J, Desco M, Green MV. Exact positioning for OSEM reconstructions on the ATLAS depth-of-interaction small animal scanner [abstract]. Mol Imaging Biol 2002;4:S22.
Liow JS, Johnson CA, Toyama H, Green MV, Innis RB. A single slice rebinning/2D exact positioning OSEM reconstruction for the NIH ATLAS small animal PET scanner [abstract]. J Nucl Med 2003;44 Suppl:P163.
George P, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998.
McLafferty FW, Turečk F. Interpretation of mass spectra. 4th ed. Mill Valley: University Science Books; 1993.
Tozuka Z, Kaneko H, Shiraga T, Mitani Y, Beppu M, Terashita S, et al. Strategy for structural elucidation of drugs and drug metabolites using (MS)n fragmentation in an electrospray ion trap. J Mass Spectrom 2003;38:793–808.
Thomas RC, Ikeda GJ. The metabolic fate of tolbutamide in man and in the rat. J Med Chem 1966;9:507–10.
Shetty HU, Nelson WL. Chemical aspects of metoprolol metabolism. Asymmetric synthesis and absolute configuration of the 3-[4-(l-hydroxy-2-methoxyethyl)phenoxy]-l-(isopropylamino)-2-propanols, the diastereomeric benzylic hydroxylation metabolites. J Med Chem 1988;31:55–9.
Andersson SHG, Lindgren A, Postlind H. Biotransformation of tolterodine, a new muscarinic receptor antagonist, in mice, rats and dogs. Drug Metab Disp 1998;26:528–35.
Hanioka H, Hamamura M, Kakino K, Ogata H, Jinno H, Takahashi A, et al. Dog liver microsomal P450 enzyme-mediated toluene biotransformation. Xenobiotica 1995;25:1207–17.
Chapman DE, Moore TJ, Michener SR, Powis G. Metabolism and covalent binding of [14C]toluene by human and rat liver microsomal fractions and liver slices. Drug Metab Disp 1990;18:929–36.
Wagner FW, Burger AR, Vallee BL. Kinetic properties of human liver alcohol dehydrogenase: oxidation of alcohols by class I isoenzymes. Biochemistry 1983;22:1857–63.
Lindahl R, Evces S. Rat liver aldehyde dehydrogenase I. Isolation and characterization of four high K m normal liver isozymes. J Biol Chem 1984;259:11986–90.
Rapoport SI, Ohno K, Pettigrew KD. Drug entry into the brain. Brain Res 1979;172:354–9.
Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J Neurochem 1998;70:1781–92.
Anandatheerthavarada HK, Boyd MR, Ravindranath V. Characterization of a phenobarbital-inducible cytochrome P-450, NADPH-cytochrome P-450 reductase and reconstituted cytochrome P-450 mono-oxygenase system from rat brain: evidence for constitutive presence in rat and human brain. Biochem J 1992;288:483–8.
Galter D, Carmine A, Buervenich S, Duester G, Olson L. Distribution of class I, III and IV alcohol dehydrogenase mRNAs in the adult rat, mouse and human brain. Eur J Biochem 2003;270:1316–26.
Erwin VG, Deitrich RA. Brain aldehyde dehydrogenase: localization, purification, and properties. J Biol Chem 1966;241:3533–9.
Acknowledgement
This work was supported by the Intramural Research Program of NIH (NIMH; project no. Z01-MH002795-04).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shetty, H.U., Zoghbi, S.S., Liow, JS. et al. Identification and regional distribution in rat brain of radiometabolites of the dopamine transporter PET radioligand [11C]PE2I. Eur J Nucl Med Mol Imaging 34, 667–678 (2007). https://doi.org/10.1007/s00259-006-0277-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0277-1