Abstract
Purpose
The purpose of this study was to evaluate and compare, by means of dynamic PET, the pharmacokinetics of 68Ga-DOTATOC, a tracer which reflects the expression of somatostatin receptors (SSTRs), and of [18F]FDG, a marker of tumour viability, in patients with metastatic neuroendocrine tumours (NETs) in whom 90Y-DOTATOC therapy was planned.
Materials and methods
Fifteen patients (63 lesions) with confirmed metastatic NETs were enrolled in this study. Dynamic [18F]FDG and 68Ga-DOTATOC PET scans were performed on two different days in the same week. The data analysis was based on qualitative and quantitative analysis using a two-tissue compartment model with a blood compartment and a non-compartment model based on the fractal dimension (FD). Multivariate analysis was used for evaluation of the kinetic data.
Results
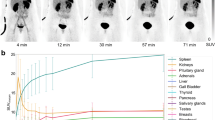
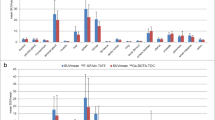
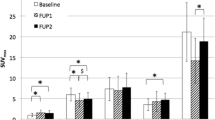
Enhanced [18F]FDG uptake was observed in 43/63 lesions. 68Ga-DOTATOC showed pathologically enhanced uptake in all evaluated patients and in 57/63 lesions. Discordant scintigraphic results for [18F]FDG and 68Ga-DOTATOC were observed in 6/15 patients. Global SUV was defined as the SUV measured in the last frame (55–60 min p.i.) of the dynamic series, for each tracer. The median global SUV uptake was 7.9 for 68Ga-DOTATOC and 4.6 for [18F]FDG. The selection of patients for 90Y-DOTATOC therapy was based on the uptake of 68Ga-DOTATOC. Multiple linear regression analysis was applied to determine the effect of each kinetic parameter (K 1–k 4, V B) on the global SUV of both tracers. The highest positive t-ratio was found for K 1 (receptor binding), followed by k 3 (cellular internalisation) and V B (fractional blood volume), when using the global 68Ga-DOTATOC uptake (SUV) as a target variable. Analysis of the [18F]FDG data revealed the highest positive t-ratio for V B, followed by k 3 (phosphorylation) and K 1 (influx). The comparison of global SUV, K 1–k 4 and the FD for [18F]FDG and 68Ga-DOTATOC did not show any statistically significant correlation. The only parameter that demonstrated a significant linear correlation between the tracers was V B.
Conclusion
68Ga-DOTATOC is a promising tool for evaluation of the expression of SSTR2 in NETs. The combination of [18F]FDG and 68Ga-DOTATOC dynamic PET studies provides different information regarding the biological properties of lesions in patients with metastatic NETs in whom 90Y-DOTATOC therapy is planned. While the global 68Ga-DOTATOC uptake is influenced mostly by K 1, the global [18F]FDG uptake is mostly influenced by V B. Only patients with enhanced 68Ga-DOTATOC uptake (SUV >5.0) were referred to 90Y-DOTATOC therapy.





Similar content being viewed by others
References
Kaltsas G, Besser GM, Grossman AB. The diagnosis and medical management of advanced neuroendocrine tumors. Endocr Rev 2004;25:458–511
Strauss LG, Conti PS. The applications of PET in clinical oncology. J Nucl Med 1991;32:623–648
Dimitrakopoulou-Strauss A, Strauss LG, Schwarzbach M, Burger C, Heichel T, Willeke F, et al. Dynamic PET 18F-FDG studies in patients with primary and recurrent soft-tissue sarcomas: impact on diagnosis and correlation with grading. J Nucl Med 2001;42:713–720
Adams S, Baum R, Rink T, Schumm-Dräger PM, Usadel KH, Hör G. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med 1998;25:79–83
Pacak K, Eisenhofer G, Goldstein DS. Functional imaging of endocrine tumors: role of positron emission tomography. Endocr Rev 2004;25:568–580
Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labelled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjugate Chem 2002;13:530–541
Reubi JC, Schär JC, Waser B, Wenger S, Heppeler A, Schmitt JS, et al. Affinity profiles for human somatostatin receptor subtypes SST1–SST5 of human somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000;27:273–282
Dimitrakopoulou-Strauss A, Strauss LG, Mikolajczyk K, Burger C, Lehnert T, Bernd L, et al. On the fractal nature of dynamic positron emission tomography (PET) studies. World J Nucl Med 2003;2:306–313
Toorongian SA, Mulholland GK, Jewett DM, Bachelor MA, Kilbourn MR. Routine production of 2-deoxy-2 (18F)fluoro-D-glucose by direct nucleophilic exchange on a quaternary 4-aminopyridinium resin. Nucl Med Biol 1990;3:273–279
Heppeler A, Froidevaux S, Eberle AN, Mäcke HR. Radiometal-labeled macrocyclic chelator-derivatised somatostatin analogue with superb tumor-targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J 1999;5:1974–1981
Schuhmacher J, Maier-Borst W. A new 68Ge/68Ga radiosotope generator system for production of 68Ga in dilute HCL. Appl Radiat Isotopes 1981;32:31–36
Kontaxakis G, Strauss LG, Thireou T, Ledesma-Carbayo MJ, Santos A, Pavlopoulos SA, et al. Iterative image reconstruction for clinical PET using ordered subsets, median root prior, and a web-based interface. Mol Imaging Biol 2002;4:219–231
Burger C, Buck A. Requirements and implementations of a flexible kinetic modeling tool. J Nucl Med 1997;38:1818–1823
Ohtake T, Kosaka N, Watanabe T, Yokoyama I, Moritan T, Masuo M, et al. Noninvasive method to obtain input function for measuring tissue glucose utilization of thoracic and abdominal organs. J Nucl Med 1991;32:1432–1438
Vesselle H, Schmidt RA, Pugsley JM, Li M, Kohlmyer SG, Vallires E, et al. Lung cancer proliferation correlates with (F-18)fluorodeoxyglucose uptake by positron emission tomography. Clin Cancer Res 2000;6:3837–3844
Foley AL, Lyles RH, Sprouse JT, Conrad EU 3rd, Eary JF. (F-18) fluorodeoxyglucose positron emission tomography as a predictor of pathologic grade and other prognostic variables in bone and soft tissue sarcoma. Clin Cancer Res 2000;6:1279–1287
Higashi K, Ueda Y, Ayabe K, Sakurai A, Seki H, Nambu Y, et al. FDG PET in the evaluation of aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun 2000;21:707–714
Belhocine T, Foidart J, Rigo P, Najjar F, Thiry A, Quatresooz P, et al. Fluorodeoxyglucose positron emission tomography and somatostatin receptor scintigraphy for diagnosing and staging carcinoid tumours: correlations with the pathological indexes p53 and Ki-67. Nucl Med Commun 2002;23:727–734
Leboulleux S, Dromain C, Bonniaud G, Auperin A, Caillou B, Lumbroso J, et al. Diagnostic and prognostic value of 18-fluorodeoxyglucose positron emission tomography in adrenocortical carcinoma: a prospective comparison with computed tomography. J Clin Endocrin Metab 2006;91:920–925
Heppeler A, Fridevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem 2000;7:971–994
Hofmann M, Maecke H, Börner AR, Weckesser E, Schoffski P, Oei L, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand 68Ga-DOTATOC: preliminary data. Eur J Nucl Med 2001;28:1751–1757
Dimitrakopoulou-Strauss A, Strauss LG, Burger C. Quantitative PET studies in pretreated melanoma patients: a comparison of 6-[18F]fluoro-L-dopa with 18F-FDG and 15O-water using compartment and noncompartment analysis. J Nucl Med 2001;42:248–256
Dimitrakopoulou-Strauss A, Strauss LG, Heichel T, Wu H, Burger C, Bernd L, et al. The role of quantitative (18F)-FDG PET studies for the differentiation of malignant and benign bone lesions. J Nucl Med 2002;43:510–518
Henze M, Dimitrakopoulou-Strauss A, Milker-Zabel S, Schuhmacher J, Strauss LG, Doll J, et al. Characterization of (68Ga)-DOTA-D-Phe1-Tyr3-octreotide (DOTATOC) kinetics in patients with meningiomas. J Nucl Med 2005;46:763–769
Dimitrakopoulou-Strauss A, Georgoulias V, Schuhmacher J, Herth F, Koukouraki S, Maecke HR, et al. Quantitative assessment of SSTR2 expression in patients with non-small cell lung cancer using 68Ga-DOTATOC PET and comparison to F-18-FDG. Eur J Nucl Med Mol Imaging 2006; in press. DOI10.1007/s00259-005-0063-5
Acknowledgement
We thank Prof. Dr. Helmut R. Mäcke (University Hospital Basel, Switzerland) for providing sample material of DOTATOC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koukouraki, S., Strauss, L.G., Georgoulias, V. et al. Comparison of the pharmacokinetics of 68Ga-DOTATOC and [18F]FDG in patients with metastatic neuroendocrine tumours scheduled for 90Y-DOTATOC therapy. Eur J Nucl Med Mol Imaging 33, 1115–1122 (2006). https://doi.org/10.1007/s00259-006-0110-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0110-x