Abstract
Purpose
As primary osseous metastasis is the main adverse prognostic factor in patients with Ewing tumours, a NOD/scid mouse model for human Ewing tumour metastases has been established to examine the mechanisms of metastasis. The aim of this study was to evaluate the feasibility of diagnostic molecular imaging by small animal PET in this mouse model.
Methods
Human Ewing tumour cells were transplanted into immune-deficient NOD/scid mice via s.c injection (n=17) or i.v. injection (n=17). The animals (mean weight 23.2 g) were studied 2–7 weeks after transplantation using a submillimetre resolution animal PET scanner. To assess glucose utilisation and bone metabolism, mice were scanned after intravenous injection of 9.6 MBq (mean) 2-[18F]fluoro-2-deoxy-D-glucose (FDG) or 9.4 MBq (mean) [18F]fluoride. Whole-body PET images were analysed visually and semi-quantitatively [%ID/g, tumour to non-tumour ratio (T/NT)]. Foci of pathological uptake were identified with respect to the physiological organ uptake in corresponding regions.
Results
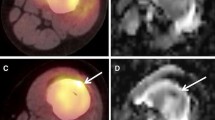
Subcutaneously transplanted Ewing tumours demonstrated a moderately increased glucose uptake (median %ID/g 2.5; median T/NT 2.2). After i.v. transplantation, the pattern of metastasis was similar to that in patients with metastases in lung, bone and soft tissue. These metastases showed an increased FDG uptake (median %ID/g 3.6; median T/NT 2.7). Osseous metastases were additionally visible on [18F]fluoride PET by virtue of decreased [18F]fluoride uptake (osteolysis; median %ID/g 8.4; median T/NT 0.59). Metastases were confirmed immunohistologically.
Conclusion
Diagnostic molecular imaging of Ewing tumours and their small metastases in an in vivo NOD/scid mouse model is feasible using a submillimetre resolution PET scanner.





Similar content being viewed by others
References
Paulussen M, Ahrens S, Dunst J, Winkelmann W, Exner GU, Kotz R, et al. Localized Ewing tumor of bone: final results on the cooperative Ewing’s sarcoma study CESS 86. J Clin Oncol 2001;19:1818–1829
Paulussen M, Ahrens S, Craft AW, Dunst J, Fröhlich B, Jabar S, et al. Ewing’s sarcoma with primary lung metastases: survival analysis of 114 (European Intergroup) Cooperative Ewing’s Sarcoma Studies patients. J Clin Oncol 1998;16:3044–3052
Paulussen M, Ahrens S, Burdach S, Craft A, Dockhorn-Dworniczak B, Dunst J, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol 1998;9:275–281
Delattre O, Zucman J, Plougastel B, Desmaze C, Melat T, Peter M, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature 1992;359:162–165
Delattre O, Zucman J, Melot T, Garau XS, Zucker JM, Lenoir GM, et al. The Ewing family of tumors—a subgroup of small-round-cell tumors defined by specific chimeric transcripts. N Engl J Med 1994;331:294–299
Dagher R, Pham TA, Sobara L, Kumar S, Long L, Bernstein D, et al. Molecular confirmation of Ewing sarcoma. J Pediatr Hematol Oncol 2001;23:221–224
Lewis JS, Achilefu S, Garbow JR, Laforest R, Welch MJ. Small animal imaging: current technology and perspectives for oncological imaging. Eur J Cancer 2002;38:2173–2188
Chatziioannou AF. Molecular imaging of small animals with dedicated PET tomographs. Eur J Nucl Med Mol Imaging 2001;29:98–114
Vormoor J, Baersch G, Decker S, Hotfilder M, Schäfer K-L, Pelken L, et al. Establishment of an in vivo model for pediatric Ewing tumors by transplantation into NOD/scid mice. Pediatr Res 2001;49:332–341
Vormoor J, Decker S, Hotfilder M, Poremba C, Dockhorn-Dwornizcak B, Jürgens H. Mechanisms of Ewing tumor cell metastasis to the bone marrow. Blood 2000;96:153b
Schäfers KP, Reader AJ, Kriens M, Knoess C, Schober O, Schäfers M. Performance evaluation of the 32-module quadHIDAC small animal PET scanner. J Nucl Med 2005;46:996–1004
Whang-Peng J, Triche TJ, Knutsen T, Miser J, Douglass EC, Israel MA. Chromosome translocation in peripheral neuroepithelioma. N Engl J Med 1984;311:584–585
van Valen F, Winkelmann W, Jürgens H. Type I and type II insulin-like growth factor receptors and their function in human Ewing’s sarcoma cells in culture. J Cancer Res Clin Oncol 1992;118:269–275
Kodama K, Doi O, Higashiyama M, Yokouchi H, Tateishi R, Mori Y. Differentiation of a Ewing’s sarcoma cell line towards neural and mesenchymal cell lineages. Jpn J Cancer Res 1994;85:335–338
Shultz LD, Schweitzer P, Christianson S, Gott B, Birdsall-Maller I, Tennent B, et al. Multiple defects ininnate and adaptive immunological function in NOD/LtSz-scid mice. J Immunol 1995;154:180–191
Wieland BW, Bida GT, Padgett HC, Go H. Current status of CTI target systems for the production of PET radiochemicals. Proceedings of the 3rd Workshop on Targetry and Target Chemistry, Vancouver, British Columbia, Canada, 19–23 June 1989
Hamacher K, Coenen HH, Stoecklin G. Efficient stereospecific synthesis of no-carrier-added 2[F-18]-fluoro-2-deoxy-D-glucose using amino polyether supported nucleophilic substitution. J Nucl Med 1986;27:235–238
Jeavons AP, Chandler RA, Deuttmar CAR. A 3D HIDAC-PET camera with submillimeter resolution for imaging small animals. IEEE Trans Nucl Sci 1999;3:468–473
Berger F, Lee Y-P, Loening AM, Chatziioannou A, Freeland SJ, Leahy R, et al. Whole-body skeletal imaging in mice utilizing microPET: optimization of reproducibility and applications in animal models of bone disease. Eur J Nucl Med Mol Imaging 2002;29:1225–1236
Moore JV, Wallner ML, Zhao S, Dodd NJF, Acton PD, Jeavons AP, et al. Feasibility of imaging photodynamic injury to tumours by high-resolution positron emission tomography. Eur J Nucl Med Mol Imaging 1998;25:1248–1254
Reader AJ, Ally S, Bakatselos F, Manavaki R, Walledge RJ, Jeavons AP, et al. One-pass list-mode EM algorithm for high resolution image reconstruction into large arrays. IEEE Trans Nucl Sci 2002;49:693–699
Palmedo H, Hensel J, Reinhardt M, von Mallek D, Matthies A, Biersack H-J. Breast cancer imaging with PET and SPECT agents: an in vivo comparison. Nucl Med Biol 2002;20:809–815
Yang H, Berger F, Tran C, Gambhir SS, Sawyers CL. MicroPET imaging of prostate cancer in LNCAP-SR39TK-GFP mouse xenografts. Prostate 2003;55:39–47
Barthel H, Cleij MC, Collingridge DR, Hutchinson OC, Osman S, He Q, et al. 3′-Deoxy-3′-[F-18]fluorothymidine as a new marker for monitoring tumor response to antiproliferative therapy in vivo with positron emission tomography. Cancer Res 2003;63:3791–3798
Boisgard R, Vincent-Naulleau S, Leplat J-J, Bouet S, Le Chalony C, Tricaud Y, et al. A new animal model for the imaging of melanoma: correlation of FDG PET with clinical outcome, macroscopic aspects and histological classification in melanoblastoma-bearing Libechav minipigs. Eur J Nucl Med Mol Imaging 2003;30:826–834
Cooper CR, Chay CH, Gendernalik JD, Lee H-L, Bhatia J, Taichman RS, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer 2003;97(Suppl 3):739–747
Burger M, Glodek A, Hartmann T, Schmitt-Gräff A, Silberstein LE, Fujii N, et al. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stroma cells. Oncogene 2003;22:8093–8101
Fernandis AZ, Prasad A, Band H, Klösel R, Ganju RK. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer. Oncogene 2004;23:157–167
Wu AM, Yazaki PJ, Tsai S, Nguyen K, Anderson A-L, McCarthy DW, et al. High-resolution microPET imaging of carcinoembryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. PNAS 2000;97:8495–8500
Franzius C, Sciuk J, Daldrup-Link HE, Jürgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumors: comparison with bone scintigraphy. Eur J Nucl Med 2000;27:1305–1311
Franzius C, Sciuk J, Brinkschmidt C, Jürgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18-FDG-PET compared with histologically assessed tumor necrosis. Clin Nucl Med 2000;25:874–881
Franzius C, Daldrup-Link HE, Sciuk J, Rummeny EJ, Bielack S, Jürgens H, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol 2001;12:479–486
Franzius C, Daldrup-Link HE, Wagner-Bohn A, Sciuk J, Heindel WL, Jürgens H, et al. FDG PET for detection of recurrences from malignant primary bone tumors: comparison with conventional imaging. Ann Oncol 2002;13:157–160
Tu S-M, Lin S-H, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumors. Lancet Oncol 2002;3:508–513
Dick JE. Breast cancer stem cells revealed. PNAS 2003;100:3547–3549
Acknowledgements
The authors gratefully acknowledge the technicians Christine Bätza and Christiane Schäfers for their technical help with the PET imaging and semi-quantitative analysis and Sabine Schneeloch and Claire Feldhoff for their technical help with the histological and immunohistological examinations. The authors thank Dr. rer. nat. Klaus Kopka and Dr. rer. nat. Stefan Wagner for the production of the radiopharmaceuticals. Furthermore, they are grateful to Marilyn Law, PhD, for reviewing the manuscript with respect to the English language. This work was supported by the Innovative Medizinische Forschung (IMF) grant (FR 210321) from the University Hospital Münster, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Franzius, C., Hotfilder, M., Poremba, C. et al. Successful high-resolution animal positron emission tomography of human Ewing tumours and their metastases in a murine xenograft model. Eur J Nucl Med Mol Imaging 33, 1432–1441 (2006). https://doi.org/10.1007/s00259-006-0106-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0106-6