Abstract
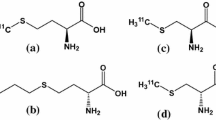
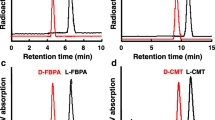
The aim of this study was to evaluate the properties of the D-amino acid isomers O-18F-fluoromethyl tyrosine (18F-FMT), O-18F-fluoroethyl tyrosine (18F-FET) and O-18F-fluoropropyl tyrosine (18F-FPT) as tumour-detecting agents with PET in comparison with the corresponding L-isomers. L- or D-18F-FMT, 18F-FET or 18F-FPT, prepared by 18F-fluoromethylation, 18F-fluoroethylation or 18F-fluoropropylation of L- and D-tyrosine, was intravenously injected into BALB/cA Jcl-nu mice bearing HeLa tumour cells. At 5, 15, 30 and 60 min post intravenous administration, the uptake of each compound in normal abdominal organs and xenotransplanted HeLa cells was determined using the tissue dissection method. Metabolic stability analyses of these compounds in the plasma were performed with the thin-layer chromatography method. In the plasma fraction, although L- and D-isomers of 18F-FMT, 18F-FET and 18F-FPT provided comparable metabolic stability, D-isomers of these labelled compounds revealed a faster elimination rate than their L-isomers, with a higher peak uptake in the blood and kidney 5 min post administration. Compared with natural amino acid ligands, such as L-11C-methionine, the uptake of L-isomers of these labelled compounds was relatively low and stable in the abdominal organs, while D-isomers revealed much lower and faster clearance rates compared with the corresponding L-isomers. Among the abdominal organs, the pancreas showed relatively high uptake of all the labelled compounds used here, and the uptake of D-isomers was much lower than that of the L-isomers. Although tumour uptake levels of D-isomers of 18F-FMT, 18F-FET and 18F-FPT were almost 95%, 43% and 39% of the uptake levels of each of the L-isomers 60 min post administration, the tumour-to-blood ratios of these D-isomers were 181%, 137% and 101% of the ratios of the corresponding L-isomers. D-isomers of 18F-FMT and 18F-FET indicated improved tumour-to-liver ratios compared with the corresponding L-isomers, and D-18F-FPT showed the highest tumour-to-pancreas ratio among all the other compounds assayed here. These results suggest that D-isomers of 18F-fluoroalkyl tyrosine analogues are potential tracers for tumour imaging with PET.




Similar content being viewed by others
References
Bolster JW, Vaalburg W, Elsinga PH, Wijnberg H, Woldring MG. Synthesis of DL-[1-11C]methionine. Appl Radiat Isot 1986;37:1069–1070
Ishiwata K, Vaalburg W, Elsinga PH, Paans AMJ, Woldring MG. Comparison of L-[1-11C]methionine and L-methyl-[11C]methionine for measuring in vivo protein synthesis rates with PET. J Nucl Med 1988;29:1419–1427
Comar D, Cartron JC, Maziere M, Marazano C. Labeling and metabolism of methionine-methyl-11C. Eur J Nucl Med 1976;1:11–14
Långström B, Antoni G, Gulberg P, Halldin C, Malmborg P, Någren K, et al. Synthesis of L and D-[methyl-11C]methionine. J Nucl Med 1987;28:1037–1040
Halldin C, Schoeps KO, Stone-Elander S, Wiesel FA. The Bucherer-Strecker synthesis of D- and L-[1-11C]tyrosine and the in vivo study of L-[1-11C]tyrosine in human brain using positron emission tomography. Eur J Nucl Med 1987;13:288–291
Ishiwata K, Vaalburg W, Elsinga PH, Paans AMJ, Woldring MG. Metabolic studies with L-[11C]tyrosine for the investigation of a kinetic model to measure protein synthesis rates with PET. J Nucl Med 1988;29:524–529
Bjurling P, Watanabe Y, Oka S, Nagasawa T, Yamada H, Långström B. Multienzymatic synthesis of β-11C-labelled L-tyrosine and L-DOPA. Acta Chem Scand 1990;44:183–188
Hawkins RA, Huang SC, Barrio JR, Keen RE, Feng D, Maziotta JC, et al. Estimation of local cerebral protein synthesis rates with L-[1-11C]leucine and PE: methods, model and results in animals and humans. J Cereb Blood Flow Metab 1989;9:446–460
Casey DL, Digenis GA, Wesner DA, Washburn LC, Chaney JE, Hayes RL, et al. Preparation and preliminary tissue studies of optically active D- and L-[11C]phenylalanine. Int J Appl Radiat Isot 1981;32:1291–1300
Barrio JR, Keen RE, Chugani H, Ackerman R, Chugani D, Phelps ME. L-[11C]Phenylalanine for the determination of cerebral protein synthesis rates in man with positron emission tomography. J Nucl Med 1983;24:70
Lemaire C, Guillaume M, Christiaens L, Palmer AJ, Cantineau R. A new route for the synthesis of [18F]fluoroaromatic substituted amino acids: no carrier acids L-p-[18F]fluorophenylalanine. Int J Radiat Appl 1987;38:1033–1038
Coenen HH, Kling P, Stöcklin G. Cerebral metabolism of L-[2-18F]fluorotyrosine, a new PET tracer of protein synthesis. J Nucl Med 1989;30:1367–1372
Wienhard K, Herholz K, Coenen HH, Rudolf J, Kling P, Stocklin G, et al. Increased amino acid transport into brain tumors measured by PET of L-(2-18F)fluorotyrosine. J Nucl Med 1991;32:1338–1346
Vaalburg W, Coenen HH, Crouzel C, Elsinga PH, Långström B, Lemaire C, et al. Amino acids for the measurement of protein synthesis in vivo by PET. Nucl Med Biol 1992;19:227–237
Iwata R, Furumoto S, Pascali C, Bogni A, Ishiwata K. Radiosynthesis of O-[11C]methyl-L-tyrosine and O-[18F]fluoromethyl-L-tyrosine as potential PET tracer for imaging amino acid transport. J Label Compd Radioparm 2003;46:555–566
Ishiwata K, Kawamura K, Wang WF, Furumoto S, Kubota K, Pascli C, et al. Evaluation of O-[11C]methyl-L-tyrosine and O-[18F]fluoromethyl-L-tyrosine as tumor imaging tracers by PET. Nucl Med Biol 2004;31:191–198
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med 1999;40:205–212
Heiss P, Mayer S, Herz M, Wester HJ, Schwaiger M, Senekowitsch-Schmidtke R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-L-tyrosine in vitro and in vivo. J Nucl Med 1999;40:1367–1373
Tang G, Tang X, Wang M, Luo L, Gan M. Fully automated synthesis of O-(3-[18F]fluoropropyl)-L-tyrosine by direct nucleophilic exchange on a quaternary 4-aminopyridium resin. Appl Radiat Isot 2003;58:685–689
Tang G, Wang M, Tang X, Luo L, Gan M. Synthesis and evaluation of O-(3-[18F]fluoropropyl)-L-tyrosine as an oncologic PET tracer. Nucl Med Biol 2003;30:733–739
Ishiwata K, Kasahara C, Hatano K, Ishii S, Senda M. Carbon-11 labeled ethionine and propionine as tumor detecting agents. Ann Nucl Med 1997;11:115–122
Takeda A, Goto R, Tamemasa O, Chaney JE, Digenis GA. Biological evaluation of radiolabeled D-methionine as a parent compound in potential nuclear imaging. Radioisotopes 1984;33:213–217
Tamemasa O, Goto R, Takeda A, Maruo K. High uptake of 14C-labeled D-amino acids by various tumors. Gann 1982;73:147–152
Tsukada H, Kengo S, Fukumoto D, Nishiyama S, Harada N, Kakiuchi T. Evaluation of D-isomers of O-11C-methyl tyrosine and O-18F-fluoromethyl tyrosine, as tumor imaging agents in tumor-bearing mice: a comparison study with L- and D-11C-methionine. J Nucl Med 2006;47:679–688
Schober O, Duden C, Meyer GJ, Muller JA, Hundeshagen H. Nonselective transport of [11C-methyl]-L- and D-methionine into a malignant glioma. Eur J Nucl Med 1987;13:103–105
Bergström M, Lundqvist H, Ericson K, Lilia A, Johanström P, Långström B, et al. Comparison of the accumulation kinetics of L-(methyl-11C)-methionine and D-(methyl-11C)-methionine in brain tumors studies with positron emission tomography. Acta Radiol 1987;28:225–229
Goto R, Tezuka M, Tamemasa O. Incorporation of L-, D-, and DL-amino acids into the pancreas of mice. Chem Pharm Bull 1977;25:1574–1581
Tamemasa O, Digenis GA, Tezuka M, Takeda A, Chaney LE, Goto R. Differential radioactivity uptake from 14C-labeled D- and L-leucine by the pancreas of animals pretreated with pancreatitis-causing agents. Chem Pharm Bull 1982;30:2521–2528
Oxender DL, Christensen HN. Distinct mediating system for the transport of natural amino acids by the Ehrich cell. J Biol Chem 1963;238:3686–3699
Christensen HN. On the development of amino acid transport system. Fed Proc 1973;32:19–28
Kubota K, Yamada K, Fukuda H, Endo S, Ito M, Abe Y, et al. Tumor detection with carbon-11-labelled amino acids. Eur J Nucl Med 1984;9:136–140
Acknowledgements
We gratefully acknowledge the excellent technical assistance provided by Shingo Nishiyama and Norihiro Harada in the synthesis of the labelled compounds. This study was supported in part by Research and Development of Technology for Measuring Vital Function Merged with Optical Technology, Research and Development Project Aimed at Economic Revitalisation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsukada, H., Sato, K., Fukumoto, D. et al. Evaluation of D-isomers of O-18F-fluoromethyl, O-18F-fluoroethyl and O-18F-fluoropropyl tyrosine as tumour imaging agents in mice. Eur J Nucl Med Mol Imaging 33, 1017–1024 (2006). https://doi.org/10.1007/s00259-006-0076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-006-0076-8