Abstract
Purpose
Several histopathological studies have demonstrated that vulnerable plaques are enriched in inflammatory cells. The aims of this study were: (1a) to test the ability of 99mTc-labelled interleukin-2 (99mTc-IL2) to bind to IL2R-positive (IL2R+) cells in carotid plaques and (1b) to correlate the plaque uptake of 99mTc-IL2, measured in vivo, with the number of IL2R+ cells within the plaque, measured ex vivo by histology (transversal study, TS), and (2) to evaluate changes in 99mTc-IL2 uptake in plaques, before and after treatment with a statin or a hypocholesterolaemic diet (longitudinal study, LS).
Methods
Ultrasound scan was performed for plaque characterisation and localisation. Fourteen patients (16 plaques) eligible for endoarterectomy were recruited for the TS and underwent 99mTc-IL2 scintigraphy before surgery. Nine patients (13 plaques) were recruited for the LS; these patients received atorvastatin or a standard hypocholesterolaemic diet and 99mTc-IL2 scintigraphy was performed before and after 3 months of treatment.
Results
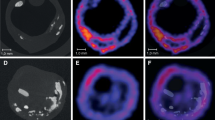
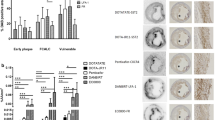
The degree of 99mTc-IL2 uptake was expressed as the plaque/background (T/B) ratio. In patients from TS, T/B ratios correlated with the percentage of IL2R+ cells at histology (r=0.707; p=0.002) and the number of IL2R+ cells at flow cytometry (r=0.711; p=0.006). No correlations were observed between ultrasound scores and either scintigraphic or histological findings. In patients from the LS, the mean 99mTc-IL2 uptake decreased in statin-treated patients (1.75±0.50 vs 2.16±0.44; p=0.012), while it was unchanged in the patients on the hypocholesterolaemic diet (2.33±0.45 vs 2.34±0.5).
Conclusion
99mTc-IL2 accumulates in vulnerable carotid plaques; this accumulation is correlated with the amount of IL2R+ cells and is influenced by lipid-lowering treatment with a statin.




Similar content being viewed by others
References
Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 1999;340:115–26
Schumacher H, Kaiser E, Schnabel PA, Sykora J, Eckstein HH, Allenberg JR. Immunophenotypic characterisation of carotid plaque: increased amount of inflammatory cells as an independent predictor for ischaemic symptoms. Eur J Vasc Endovasc Surg 2001;1:494–501
Spagnoli LG, Mauriello A, Sangiorgi G, Fratoni S, Bonanno E, Schwartz RS, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004;92:1845–52
Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;13:1315–22
Carr SC, Farb A, Pearce WH, Virmani R, Yao JS. Activated inflammatory cells are associated with plaque rupture in carotid artery stenosis. Surgery 1997;122:757–63
Spagnoli LG, Bonanno E, Mauriello A, Palmieri G, Partenzi A, Sangiorgi G, et al. Multicentric inflammation in epicardial coronary arteries of patients dying of acute myocardial infarction. J Am Coll Cardiol 2002;40:1579–88
Schönbeck U, Mach F, Sukhova GK, Murphy C, Bonnefoy JY, Fabunmi RP, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res 1997;81:448–54
Yuan C, Tsuruda JS, Beach KN, Hayes CE, Ferguson MS, Alpers CE, et al. Techniques for high resolution MR imaging of atherosclerotic plaque. J Magn Reson Imaging 1994;4:43–9
Virgolini I, Rauscha F, Lupattelli G, Angelberger P, Ventura A, O’Grady J, et al. Autologous low-density lipoprotein labelling allows characterization of human atherosclerotic lesions in vivo as to presence of foam cells and endothelial coverage. Eur J Nucl Med 1991;18:948–51
Iuliano L, Signore A, Vallabhajosula S, Colavita AR, Camastra C, Ronga G, et al. Preparation and biodistribution of 99m technetium-labelled oxidized LDL in man. Atherosclerosis 1996;126:131–41
Leitha T, Staudenherz A, Gmeiner B, Hermann M, Huttinger M, Dudczak R. Technetium-99m labelled LDL as a tracer for quantitative LDL scintigraphy. II. In vivo validation, LDL receptor-dependent and unspecific hepatic uptake and scintigraphic results. Eur J Nucl Med 1993;20:674–9
Iuliano L, Signore A, Violi F. Uptake of oxidized LDL by human atherosclerotic plaque. Circulation 1997;96:2093–4
Ogawa M, Ishino S, Mukai T, Asano D, Teramoto N, Watabe H, et al. 18F-FDG accumulation in atherosclerotic plaques: immunohistochemical and PET imaging study. J Nucl Med 2004;45:1245–50
Hanif MZ, Ghesani M, Shah AA, Kasai T. F-18 fluorodeoxyglucose uptake in atherosclerotic plaque in the mediastinum mimicking malignancy: another potential for error. Clin Nucl Med 2004;29:93–5
Tatsumi M, Cohade C, Nakamoto Y, Wahl RL. Fluorodeoxyglucose uptake in the aortic wall at PET/CT: possible finding for active atherosclerosis. Radiology 2003;229:831–7
Rudd JH, Warburton EA, Fryer TD, Jones HA, Clark JC, Antoun N, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography (symptomatic, unstable plaques accumulate more 18FDG than asymptomatic lesions). Circulation 2002;105:2708–11
Yun M, Jang S, Cucchiara A, Newberg AB, Alavi A. 18F FDG uptake in the large arteries: a correlation study with the atherogenic risk factors (cc age/chol and FDG). Semin Nucl Med 2002;32:70–6
Lederman RJ, Raylman RR, Fisher SJ, Kison PV, San H, Nabel EG, et al. Detection of atherosclerosis using a novel positron-sensitive probe and 18-fluorodeoxyglucose (FDG) (cc SMC/Mac and FDG). Nucl Med Commun 2001;22:747–53
Kolodgie FD, Petrov A, Virmani R, Narula N, Verjans JW, Weber DK, et al. Targeting of apoptotic macrophages and experimental atheroma with radiolabeled annexin V: a technique with potential for noninvasive imaging of vulnerable plaque. Circulation 2003;108:3134–9
Prat L, Torres G, Carrió I, Roca M, Riambau V, Berna L, et al. Polyclonal 111In-IgG, 125I-LDL and 125I-endothelin-1 accumulation in experimental arterial wall injury. Eur J Nucl Med 1993;20:1141–5
Qin G, Zhang Y, Cao W, An R, Gao Z, Li G, et al. Molecular imaging of atherosclerotic plaques with technetium-99m-labelled antisense oligonucleotides. Eur J Nucl Med Mol Imaging 2005;32:6–14
Knight LC. Non-oncologic applications of radiolabeled peptides in nuclear medicine. Q J Nucl Med 2003;47:279–91
Robb RJ, Greene WC, Rusk CM. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med 1984;160:1126–46
Signore A, Chianelli M, Annovazzi A, Rossi M, Maiuri L, Greco M, et al. Imaging of active lymphocytic infiltration in coeliac disease with 123I-Interleukin-2 and its response to diet. Eur J Nucl Med 2000;27:18–24
Annovazzi A, Biancone L, Caviglia R, Chianelli M, Capriotti G, Mather SJ, et al. 99mTc-Interleukin-2 and 99mTc-HMPAO granulocyte scintigraphy in patients with inactive Crohn’s disease. Eur J Nucl Med Mol Imaging 2003;30:374–82
Consensus sur la morphologie et le risque des plaques carotidiennes. A paraître dans Cerebro Vascular Disease. Basel: Karger;1997:N° 3
Chianelli M, Signore A, Fritzberg A, Mather SJ. 99mTc-interleukin-2: the development of technetium-99m labelled Interleukin 2: a new radiopharmaceutical for in vivo detection of mononuclear cell infiltrates in immune mediate diseases. Nucl Med Biol 1997;24:579–86
Bonanno E, Mauriello A, Partenzi A, Anemona L, Spagnoli LG. Flow cytometry analysis of atherosclerotic plaque cells from human carotids: a validation study. Cytometry 2000;39:158–65
Chou ET, Minutello RM, Parikh M, Bergman G, Chiu Wong S, Hong MK. Can we identify vulnerable patients at risk for ST-segment elevation myocardial infarction based on their clinical characteristics? Coron Artery Dis 2004;15:467–69
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–53
European Carotid Surgery Trialists’ Collaborative Group. Endarterectomy for moderate symptomatic carotid stenosis: interim results from the MRC European Carotid Surgery Trial. Lancet 1996;347:1591–3
Hakonarson H, Kim C, Whelan R, Campbell D, Grunstein MM. Bi-directional activation between human airway smooth muscle cells and T lymphocytes: role in induction of altered airway responsiveness. J Immunol 2001;166:293–303
Miller DD, Karim MA, Edwards WD, Schwartz RS. Relationship of vascular thrombosis and inflammatory leukocyte infiltration to neointimal growth following porcine coronary artery stent placement. Atherosclerosis 1996;124:145–55
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part I. Circulation 2003;108:1664–72
Sukhova GK, Williams JK, Libby P. Statins reduce inflammation in atheroma of nonhuman primates independent of effects on serum cholesterol. Arterioscler Thromb Vasc Biol 2002;22:1452–8
Acknowledgements
This study was partially supported by the Italian Ministry of Public Education, by the International Atomic Energy Agency (IAEA) and by GE-Amersham Healthcare. This paper was awarded with the 2004 Marie Curie award by the European Association of Nuclear Medicine (EANM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Annovazzi, A., Bonanno, E., Arca, M. et al. 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging 33, 117–126 (2006). https://doi.org/10.1007/s00259-005-1899-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-1899-4