Abstract
Purpose
Intracavitary radioimmunotherapy (RIT) offers an effective adjuvant therapeutic approach in patients with malignant gliomas. Since differentiation between recurrence and reactive changes following RIT has a critical impact on patient management, the aim of this study was to analyse the value of serial O-(2-[18F]fluoroethyl)-l-tyrosine (FET) PET scans in monitoring the effects of this locoregional treatment.
Methods
Following conventional therapy, 24 glioma patients (5 WHO III, 19 WHO IV) underwent one to five RIT cycles with either 131I-labelled (n=19) or 188Re-labelled (n=5) anti-tenascin antibodies. Patients were monitored with serial FET PET scans (2–12 scans). For semiquantitative evaluation, maximal tumoural uptake (TUmax) was evaluated and the ratio to background (BG) was calculated. Results of PET were correlated with histopathological findings (n=9) and long-term clinical follow-up for up to 87 months.
Results
In seven tumour-free patients, PET revealed slightly increasing but homogeneous FET uptake surrounding the resection cavity with a peak up to 18 months following RIT (TUmax/BG 2.07±0.25) but stable or decreasing values during further follow-up (last follow-up: TUmax/BG 1.63±0.22). Seventeen patients developed regrowth of residual tumour/tumour recurrence showing additional nodular FET uptake (TUmax/BG 2.79±0.53). A threshold value of 2.4 (TUmax/BG) allowed best differentiation between recurrence and reactive changes (sensitivity 82%, specificity 100%).
Conclusion
FET PET is a sensitive tool for monitoring the effects of locoregional RIT. Homogeneous, slightly increasing FET uptake around the tumour cavity with a peak up to 18 months after RIT, followed by stable or decreasing uptake, points to benign, therapy-related changes. In contrast, nodular uptake is a reliable indicator of recurrence.
Similar content being viewed by others
Introduction
The life expectancy of patients with high-grade gliomas, in particular glioblastoma multiforme, is still very poor. Standard treatment including surgery, radiation therapy and, if suitable, systemic chemotherapy results in median survival times ranging between 1 year for glioblastoma and about 3 years for anaplastic astrocytoma [1, 2]. Standard treatment is not able to control tumour progression for a longer period since in most cases microscopic tumour cell clusters located in the peritumoural brain tissue are left and become the starting point for (usually) early tumour recurrence [3, 4]: complete surgical resection or eradication by subsequent conventional radiation therapy is usually impossible. To overcome these limitations, more specific approaches for brain tumour treatment have been introduced, such as additional targeted radiotherapy, e.g. in form of a locoregional radioimmunotherapy (RIT) [5–9]. The infusion of radiolabelled monoclonal antibodies directly into the postsurgical resection cavity after surgery has enabled the delivery of high radiation doses to the affected area without harming the surrounding normal brain tissue or distant organs.
Treatment efficacy can be evaluated by different neuro-imaging modalities. Conventional magnetic resonance imaging (MRI) and computed tomography (CT) assess morphological parameters such as changes in tumour size, oedema or contrast enhancement. However, these changes and alterations in blood-brain barrier properties are not always related to tumour regrowth; rather, they can also represent non-specific inflammatory treatment effects and radiation or tumour necrosis, especially after locoregional administration of high-dose radiation [10–12]. Functional imaging methods have been proposed as an alternative for monitoring treatment and are claimed to be more specific than structural imaging methods.
Positron emission tomography (PET) using radiolabelled amino acid analogues, particularly [methyl-11C]-l-methionine (MET) and, in recent years, O-(2-[18F]fluoroethyl)-l-tyrosine (FET), has been employed in numerous metabolic studies of malignant brain tumours [13–21]. Since amino acid uptake has been shown to be increased relative to normal brain tissue in most low- and high-grade tumours, it has gained an important role in the diagnostic work-up of brain tumours [21–24]. PET using MET or FET has also been shown to differentiate reliably between tumour recurrence and post-therapeutic benign lesions after standard treatment modalities like surgery, external radiation therapy or chemotherapy [15, 16, 20, 25–28]. In a recent study, FET PET was evaluated for the first time in a novel locoregional therapeutic approach, for the purpose of monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Preliminary data in a small patient group suggested that FET PET seems to fulfil the clinical requirement for a method that is sensitive enough to assess therapeutic effects early and to distinguish between harmless post-therapeutic changes and tumour regrowth after this aggressive locoregional approach [29].
The aim of the present study was to evaluate the diagnostic value of FET PET in another locoregional approach, namely in monitoring the therapeutic effects of locoregional RIT following intracavitary administration of 131I- or 188Re-labelled monoclonal anti-tenascin antibodies (TN-mAb).
Materials and methods
Patients
Twenty-four patients (9 females, 15 males) with a mean age of 49±14 years and histopathologically proven tenascin expression of malignant gliomas (5 anaplastic astrocytomas, 19 glioblastomas) were included in the study. All patients had undergone primary surgery on the tumour or re-operation of tumour recurrence with implantation of an Ommaya reservoir into the resection cavity. Primary surgery had been followed in 23 of the 24 patients by external radiation therapy and in six patients by additional systemic chemotherapy.
In 7 of the 24 patients, small residual tumours with an average diameter of less than 1 cm had to be left because of their location in high-risk and functionally important areas. In 17 patients no obvious tumour was seen on baseline MRI examinations. Patients underwent one to five cycles of intracavitary RIT with 131I-labelled (n=19) or 188Re-labelled (n=5) murine TN-mAb (BC4, which is directed against the EGF-like repeats of human TN) at 6- to 8-week intervals (maximal cumulative activity 5,740 MBq for 131I and 470 MBq for 188Re). In 20 patients, serial FET PET and MRI scans were performed preceding every RIT cycle and at 3-month intervals after the end of RIT in order to detect tumour recurrence and to monitor the therapeutic effects. In the remaining four patients, follow-up with serial FET PET scans was not started until 10, 12, 17 and 33 months after the end of RIT. Altogether, a total of 119 PET scans were performed.
PET studies
FET PET scans were obtained with a Siemens ECAT EXACT HR+ scanner. To obtain standardised metabolic conditions, patients fasted for a minimum of 6 h before performing the PET scan. The scanner acquires 63 contiguous transaxial planes, simultaneously covering 15.5 cm of axial field of view. After a 15-min transmission scan (68Ge sources), 180 MBq [18F]FET was injected intravenously. Since FET accumulates and reaches a peak in malignant gliomas within 15–20 min after injection and only slowly decreases afterwards [14], a static PET study from 20 to 50 min post injection (three added frames of 10 min each, 128×128 matrix, 3D acquisition) was acquired to obtain adequate count rate statistics. Images were reconstructed by filtered backprojection using a Hann filter with a cutoff frequency of 0.5 Nyquist and corrected for scatter and attenuation. For semiquantitative evaluation, data were transferred to a HERMES work station (Hermes Medical Solutions, Sweden) and the slice with the highest FET uptake around the former resection cavity was evaluated. For this slice, maximal uptake (maximal counts within the tumour: TUmax) was determined and the ratio to the background (BG) was calculated. For background information, the mean uptake within a 70% isocontour threshold ROI mirrored to the opposite non-tumour-bearing hemisphere was determined.
Statistical analysis
The diagnostic performance in differentiating between recurrence and tumour-free status was assessed by receiver operating characteristic (ROC) analyses [30, 31]. For this purpose, data of serial PET scans were separately categorised into true- and false-positive and true- and false-negative findings using clinical follow-up or the results of histopathology as the gold standard. Then sensitivity (SN) and specificity (SP) pairs as a function of the respective decision thresholds were plotted as ROC curves to describe the inherent discrimination capacity of the diagnostic system and to define the optimal threshold for decision making. The threshold was considered optimal when the sum of paired values for SN and SP reached a maximum in two evaluations: the analysis of all performed scans (n=119) and the analysis of only that scan of each patient which presented with the highest uptake value during the entire follow-up period.
In addition, SN and SP values are given for a visual analysis using nodular versus non-nodular FET uptake as the criterion to distinguish between patients with tumour recurrence and tumour-free patients.
The duration of progression-free survival after the first RIT cycle was computed according to the Kaplan-Meier method using the SPSS for Windows (SPSS, Version 13.0, Chicago, IL) statistics package. Two groups were compared, those with maximal individual TUmax/BG ratios above ≥2.4 and those with ratios below <2.4, which was considered the threshold value for best differentiation between recurrent tumour and reactive post-therapeutic changes. Statistical differences between the two curves were tested using log rank and Breslow tests.
Results
Our clinical experience with RIT has been reported previously. We observed substantially prolonged survival times for patients who received RIT (n=37: a larger number than in this study) compared with a similarly treated group without additional RIT [5]. Therefore, readers are referred to this previous article for information on the clinical findings associated with this form of therapy; here we shall focus on the role of FET PET during follow-up only.
During the study period, 17 of the 24 patients ultimately presented with tumour progression while seven were considered tumour free. For the latter group, Table 1 summarises patient characteristics, number and time points of RIT cycles and cumulative activity, number of serial PET scans with FET uptake ratios at baseline and last follow-up, and patient survival after initial surgery. The tumour-free status was documented by biopsy in two patients (these patients presented with contrast enhancement on MRI which was highly suspicious for recurrence) and by stable clinical follow-up for between 23 and 87 months after initial surgery in the other five. Six of these seven patients demonstrated slightly increasing homogeneous FET uptake surrounding the resection cavity without any signs of additional nodular uptake; this uptake presented with a peak (baseline TUmax/BG 1.78±0.30, peak TUmax/BG 2.07±0.25, mean±SD) up to 18 months after RIT, followed by stable or decreasing values (last follow-up TUmax/BG 1.63±0.22; representative example in Fig. 1). One of these six patients unfortunately decided to discontinue the follow-up with FET PET 4 months after RIT. However, this patient was clinically followed and showed a stable course over 27 months after RIT and 35 months after initial surgery. In one of the seven patients with slightly increased stable and homogeneous FET uptake, RIT had been completed about 3 years prior to the beginning of FET PET monitoring; therefore the post-therapeutic phase of increasing FET uptake and its peak were probably missed. The peak values of TUmax/BG observed in tumour-free patients corresponded to the threshold value of 2.4 for TUmax/BG ratio as assessed by ROC analyses which allowed best differentiation between post-therapeutic reactive changes and tumour recurrence. In this small patient group, the extent and changes of FET uptake over time following RIT seemed to be independent of the delivered radiation dose since the peak TUmax/BG ratio reached similar levels in all patients. Furthermore, the time point of the peak uptake, which ranged between 2 and 18 months, also showed no obvious relation with the dose administered.
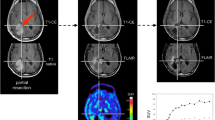
Serial MRI and FET PET scans of a 36-year-old woman after surgery for an anaplastic astrocytoma WHO III and following two RIT cycles (10/99 and 11/99 with 2,460 MBq 131I-TN-mAb). The patient was clinically tumour free at follow-up for up to 77 months after surgery. Slight linear contrast enhancement surrounding the small tumour cavity in the right central area is observed on MRI. On FET PET there is slightly increasing homogeneous FET uptake surrounding the cavity (which in this particular case mimics a more focal appearance owing to the small lesion size in combination with the limited resolution of the PET scanner), peaking 15 months after RIT (02/01) and decreasing during further follow-up. The respective TUmax/BG ratios are given below the PET images
Among the 17 of 24 patients who ultimately presented with tumour progression, ten experienced tumour recurrence and seven, regrowth of residual tumour. These diagnoses were histopathologically proven by stereotactic biopsy in five patients and by re-operation in three patients. In the remaining nine patients, suspected tumour regrowth or recurrence was assumed because of concordantly increasing contrast enhancement on MRI and clinical deterioration. Table 2 summarises the characteristics and results of patients with progressive disease. The ten patients without obvious tumour at baseline initially presented with normal ratios (TUmax/BG 1.64±0.26) but showed focally increasing FET uptake values, resulting in pathological ratios during follow-up (TUmax/BG 2.67±0.43). A representative example is shown in Fig. 2. In seven patients with known residual tumour on the baseline scan, further FET PET investigations demonstrated pathological ratios which were stable or increased over time (baseline TUmax/BG 2.49±0.42; last follow-up TUmax/BG 3.04±0.49). Six of these seven patients showed pathological contrast enhancement on baseline MRI as well. Most interestingly, in one of the seven patients (with anaplastic astrocytoma WHO grade III), MRI was rated as normal owing to the lack of contrast enhancement, whereas the concomitant PET scan showed pathological focal FET uptake ventral of the cavity, clearly indicating recurrence. This was proven by stereotactic biopsy. This patient underwent re-operation of this area, but unfortunately developed another tumour manifestation distant from the primary location that showed contrast enhancement on MRI as well (Fig. 3).
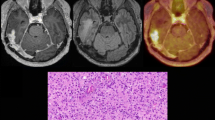
Serial MRI and FET PET images of a 69-year-old man after surgery and radiation therapy of a glioblastoma located in the left occipital lobe during and after two RIT cycles (07/04 and 10/04 with 470 MBq 188Re-TN-mAb). Slightly increased homogeneous FET uptake is evident surrounding the tumour cavity (07/04–12/04). Development of tumour recurrence lateral to the resection cavity is indicated by additional nodular FET uptake as shown in the last scan (03/05). The respective TUmax/BG ratios are given below the PET images
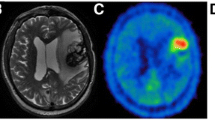
Serial MRI and FET PET scans of a 30-year-old man after surgery and radiation therapy of an anaplastic astrocytoma WHO III located in the right parietal lobe before and after 1 RIT cycle (07/02 with 1870 MBq 131I-TN-mAk). a At baseline and 3 months after therapy, slightly increased homogeneous FET uptake at the cavity borders with additional nodular FET uptake ventral of the cavity indicating recurrence. MRI shows no suspicious contrast enhancement. b After reoperation development of another tumour manifestation developed distant the primary location with focal FET uptake and contrast enhancement on MRI as well. The respective TUmax/BG ratios are given below the PET images
In total, 119 PET scans were performed, 34 in the presence of recurrent tumours and 85 while the patient had a tumour-free status. ROC analyses based on two approaches—(a) including all performed PET scans (Fig. 4a) and (b) considering only the PET scan with the highest individual uptake in each patient during follow-up (n=24) (Fig. 4b)—demonstrated the highest discrimination capacity between patients with tumour recurrence and tumour-free patients at a threshold value of 2.4 for the TUmax/BG ratio.
ROC curves illustrating the diagnostic performance of FET PET when varying the decision thresholds. Analyses were performed twice: for all available PET scans (n=119, including 85 scans performed while patients had a tumour-free status and 34 scans in patients with recurrent tumours) (a) and for only that scan presenting with the highest individual uptake (n=24) (b). The sensitivity and specificity pairs for the threshold values between 2.0 and 2.5 are given in the boxes
Comparison of patients with ratios below and above this threshold using Kaplan-Meier curves for progression-free survival (Fig. 5) demonstrated that survival times were significantly longer (p<0.05) in patients presenting with values below this threshold.
Visual analysis using a nodular versus a non-nodular uptake pattern as the criterion to distinguish between tumour recurrence and tumour-free status led to correct detection of recurrence in 32 of 34 scans (sensitivity 94%). In 80 of 85 scans performed in a tumour-free status, no additional nodular uptake pattern was present (specificity 94%). In five scans, uptake appeared somewhat focally accentuated owing to circumscribed folding of the cavity wall, and thus mimicked nodular uptake. Restricting visual analysis only to the one scan per patient presenting with the highest FET uptake resulted in correct detection of tumour recurrence in 16 of 17 patients (sensitivity 94%) and of a tumour-free status in five of seven (specificity 71%). In two of seven tumour-free patients, FET uptake that appeared nodular was misleading for reasons mentioned above.
Discussion
Recent studies on locoregional RIT have demonstrated that local administration of radiolabelled monoclonal antibodies may significantly prolong survival times in patients with malignant gliomas [5–9]. The high locally achieved radiation dose, however, may cause inflammatory infiltrates and radiation necrosis more frequently than do standard treatment modalities, rendering differential diagnosis between benign reactive post-therapeutic effects and tumour recurrence more difficult. In particular, abnormal contrast enhancement in necrotic tissue often mimics tumour recurrence and, therefore, neither MRI nor CT allows reliable distinction between tumour and reactive changes in contrast-enhancing lesions [10–12]. Due to the poor prognosis of patients with glioma, however, knowledge of whether RIT has been successful or not is required so that a decision can be made to re-operate or to change to alternative aggressive treatment modalities when necessary.
So far, to our knowledge only one study has reported on results of functional imaging following RIT of glioma. Using PET with FDG as the metabolic marker, Marriott et al. [32] reported in a limited number of ten patients that a metabolic rim of FDG was observed around the tumour cavity after intracavitary administration of 131I-labelled monoclonal antibodies. Based on their findings they suggested that the development of such a rim may be dose dependent but independent of malignant disease. Histologically, the metabolic rim was associated with an inflammatory infiltrate consisting of a relatively increased number of macrophages and fibroblasts. Furthermore, malignant recurrence was suggested in the event of development of new nodularity in the non-malignant FDG-accumulating rim.
To our knowledge, there have been no previous reports of experience with PET and amino acid tracers as metabolic markers in the special setting of locoregional RIT. A recent study that monitored a limited number of patients with FET PET after another locoregional treatment of glioma, namely convection-enhanced delivery of paclitaxel, suggested that FET PET may be more reliable than MRI in differentiating reactive post-therapeutic changes in stable disease from tumour progression [29]. These results strongly encourage the evaluation of FET PET for monitoring of other forms of locoregional glioma therapy.
This is the first study to investigate the use of an amino acid tracer to monitor the effects of locoregional administration of radiolabelled monoclonal antibodies. Twenty-four patients with malignant gliomas were followed up with serial FET PET scans during and/or after intracavitary RIT. Similar to the results reported by Marriott et al. [32], in all cases, even those considered tumour free, PET demonstrated slight homogeneous FET uptake surrounding the resection cavity, which was considered to be a consequence of therapy. However, the results of in vitro and animal studies on the uptake of FET in soft tissue infection [33] or cerebral radiation injury [34] suggest that FET uptake is probably to be explained by a breakdown in the blood-brain barrier rather than by active uptake in macrophages or inflammatory infiltrates, as in the case of FDG. This is supported by the following four observations: Breakdown of the blood-brain barrier following RIT was documented in all patients by a linear contrast-enhancing rim surrounding the resection cavity on MRI scans. Kaim et al. demonstrated that FET uptake was not increased in activated white blood cells of experimental soft tissue infection [33]. In rats, comparison of tracer accumulation in cryolesions characterised by the absence of inflammatory cells and radiation injury demonstrated that slight FET uptake was most likely due to a disruption of the blood-brain barrier and not additional trapping by macrophages. Uptake in radiation injury has generally been reported to be lower than uptake in tumours, suggesting that FET may be superior to FDG for distinguishing radiation necrosis from tumour recurrence [34].
In general, the homogeneous FET uptake around the resection cavity after RIT was somewhat higher than the unspecific uptake observed in a larger patient group after surgery and conventional external radiation therapy [20]. For these standard treatment modalities, the preliminary threshold value for best differentiation between post-therapeutic effects and tumour recurrence has been considered to be 2.0 for the TUmax/BG ratio, whereas in the present study following RIT the threshold value was determined to be 2.4. The somewhat higher unspecific FET uptake after RIT may be explained by a more severe breakdown of the blood-brain barrier, probably induced by the higher local radiation dose. However, based on the limited experience so far, we were not able to document a relationship between extent and time course of FET uptake with the dose administered.
In addition to these general considerations regarding the rim uptake, the time course of FET uptake in relation to the RIT cycles also has to be taken into account. In the seven patients without clinical signs of tumour recurrence, PET demonstrated slightly increasing values initially, with a peak at up to 18 months after RIT, and stable or even decreasing values during further follow-up. In these patients, FET uptake was homogeneous and the TUmax/BG ratio reached the threshold value of 2.4 in only one patient and then in only one (peak) of nine serial scans. In contrast, in the 17 patients presenting with tumour progression during follow-up, PET showed a more focally configured FET uptake in addition to the rim uptake. Semiquantitative evaluation of the slice with the highest uptake showed either stably increased values (in patients with residual tumour) or increasing values, starting from normal ratios and progressing to pathological ratios(in patients who developed recurrence).
Using the threshold value of 2.4 (as determined by ROC analyses) and regardless of the uptake pattern, FET PET was able to distinguish with high discriminatory power between tumour progression during or after RIT and therapy-induced benign changes: in 21/24 patients there was accurate assessment in all serial PET scans, while in the remaining three patients there was false negative assessment, with TUmax/BG ratios of 2.0, 2.1 and 2.2 respectively. However, in all three cases the nodular configuration of FET uptake led to the correct diagnosis. Even in one patient without focal contrast enhancement on MRI, PET showed focally increased FET uptake clearly indicating tumour regrowth, which was later proven by biopsy. Incidentally, this case supports the hypothesis that tumoural uptake independent of blood-brain barrier disruption is mainly mediated by active transport via the transport system L [35–37]. Visual assessment based solely on the FET uptake pattern seemed to provide slightly more sensitive results than those obtained by the ratio method, but at a lower specificity. Both the TUmax/BG ratio and the configuration of FET uptake should be taken into account in order to differentiate optimally between recurrence and benign post-therapeutic effects. If findings are then still considered equivocal, a shorter time interval between follow-up scans (e.g. 6 weeks) offers another option to achieve reliable diagnostic assessment as early as possible.
Even though it was not the aim of this study to assess the predictive value of FET PET for clinical outcome, FET uptake values following RIT could have been used to compare Kaplan-Meier-overall survival curves of patients with ratios below and above the suggested threshold value (TUmax/BG<2.4). This approach, however, was not followed in our study owing to the heterogeneous patient group (patients with and without residual tumour at baseline, various intervals between initial surgery and radioimmunotherapy, various starting points of FET PET monitoring). Instead, a Kaplan-Meier analysis for progression-free survival following RIT was performed, showing significantly longer progression-free survival in patients in whom the maximal individual uptake was below the suggested threshold value, compared to those presenting with values above this threshold.
One possible limitation of the present study was the lack of histological confirmation in 15/24 patients, and especially in five of the seven patients who were considered tumour free. Histopathological confirmation would have been ethically hard to justify in these patients, but its lack gives rise to the question of possible false negative findings. Nevertheless, the unsuspicious long-term clinical follow-up in the patients in question and the fact that the study population consisted only of patients with high-grade gliomas, and in particular glioblastomas (19/24 patients), strongly support the PET results.
Another possible limitation is that in four of the 24 patients, follow-up with serial FET PET scans did not start until at least 10 months after the end of RIT, with the consequence that some earlier changes in FET uptake following RIT might have been missed. However, since in three of those four patients FET uptake was still increasing during the initial PET scans, followed by a peak and subsequent decrease, it is unlikely that crucial information was missed. Only in one patient in whom RIT had been completed as long as 33 months prior to the beginning of FET PET monitoring were the post-therapeutic phase of increasing FET uptake and its peak probably missed.
Finally, in the present study we abstained from systematically correlating FET PET results with MRI data because the primary goal was to evaluate the value of serial FET PET scans for monitoring the effects of intracavitary RIT. Direct correlations between FET PET and MRI in a similar series of patients following multimodal systemic and/or locoregional treatment have been published recently [38], showing that MRI is insufficient to distinguish between benign side-effects of therapy and tumour recurrence and that FET PET is a powerful tool to improve the differential diagnosis in these patients. Therefore, FET PET might be a method of choice for monitoring locoregional forms of treatment, like RIT, which frequently cause treatment-related inflammatory reactions or tumour necrosis.
Conclusion
This study indicates that FET PET is a sensitive tool for monitoring the effects of high local radiation doses given by intracavitary RIT. Homogeneous, slightly increasing FET uptake around the tumour cavity with a peak up to 18 months after RIT, followed by stable or decreasing FET uptake, is indicative of benign, therapy-related changes. These findings are independent of tumour recurrence and must not be misinterpreted as progressive disease. In contrast, focally increased FET uptake is an early and reliable indicator of tumour progression.
Since both radiation necrosis and active tumour growth can present with clinical deterioration and are often impossible to distinguish by means of structural brain imaging, FET PET may be a powerful tool for planning further patient management. While radiation necrosis may be treated by steroids or, in extensive cases, by debulking surgery, recurrent tumour requires change of ineffective treatment or palliative care only.
References
Davis FG, Freels S, Grutsch J, Barlas S, Brem S. Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on surveillance, epidemiology, and end results (SEER) data, 1973–1991. J Neurosurg 1998;88:1–10
DeAngelis LM. Brain tumors. N Engl J Med 2001;344:114–123
Harbaugh KS, Black PM. Strategies in the surgical management of malignant gliomas. Semin Surg Oncol 1998;14:26–33
Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg 1993;78:767–775
Goetz C, Riva P, Poepperl G, Gildehaus FJ, Hischa A, Tatsch K, et al. Locoregional radioimmunotherapy in selected patients with malignant glioma: experiences, side effects and survival times. J Neurooncol 2003;62:321–328
Popperl G, Gotz C, Gildehaus FJ, Yousry TA, Reulen HJ, Hahn K, et al. [Initial experiences with adjuvant locoregional radioimmunotherapy using 131I-labeled monoclonal antibodies against tenascin (BC-4) for treatment of glioma (WHO III and IV)]. Nuklearmedizin 2002;41:120–128
Riva P, Franceschi G, Riva N, Casi M, Santimaria M, Adamo M. Role of nuclear medicine in the treatment of malignant gliomas: the locoregional radioimmunotherapy approach. Eur J Nucl Med 2000;27:601–609
Riva P, Franceschi G, Arista A, Frattarelli M, Riva N, Cremonini AM, et al. Local application of radiolabeled monoclonal antibodies in the treatment of high grade malignant gliomas: a six-year clinical experience. Cancer 1997;80:2733–2742
Zalutsky MR. Targeted radiotherapy of brain tumours. Br J Cancer 2004;90:1469–1473
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 2000;217:377–384
Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol 1991;12:45–62
Earnest Ft, Kelly PJ, Scheithauer BW, Kall BA, Cascino TL, Ehman RL, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 1988;166:823–827
Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–445
Weber WA, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ, et al. O-(2-[18F]fluoroethyl)-L-tyrosine and L-[methyl-11C]methionine uptake in brain tumours: initial results of a comparative study. Eur J Nucl Med 2000;27:542–549
Voges J, Herholz K, Holzer T, Wurker M, Bauer B, Pietrzyk U, et al. 11C-methionine and 18F-2-fluorodeoxyglucose positron emission tomography: a tool for diagnosis of cerebral glioma and monitoring after brachytherapy with 125I seeds. Stereotact Funct Neurosurg 1997;69:129–135
Ogawa T, Inugami A, Hatazawa J, Kanno I, Murakami M, Yasui N, et al. Clinical positron emission tomography for brain tumors: comparison of fludeoxyglucose F 18 and L-methyl-11C-methionine. AJNR Am J Neuroradiol 1996;17:345–353
Derlon JM, Bourdet C, Bustany P, Chatel M, Theron J, Darcel F, et al. [11C]L-methionine uptake in gliomas. Neurosurgery 1989;25:720–728
Schaller B. Usefulness of positron emission tomography in diagnosis and treatment follow-up of brain tumors. Neurobiol Dis 2004;15:437–448
Pauleit D, Floeth F, Hamacher K, Riemenschneider MJ, Reifenberger G, Muller HW, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain 2005;128:678–687
Popperl G, Gotz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value of O-(2-[18F]fluoroethyl)-l-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 2004;31:1464–1470
Weckesser M, Langen KJ, Rickert CH, Kloska S, Straeter R, Hamacher K, et al. O-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging 2005;32:422–429
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging 2002;29:176–182
Goldman S, Levivier M, Pirotte B, Brucher JM, Wikler D, Damhaut P, et al. Regional methionine and glucose uptake in high-grade gliomas: a comparative study on PET-guided stereotactic biopsy. J Nucl Med 1997;38:1459–1462
Ogawa T, Shishido F, Kanno I, Inugami A, Fujita H, Murakami M, et al. Cerebral glioma: evaluation with methionine PET. Radiology 1993;186:45–53
Herholz K, Kracht LW, Heiss WD. Monitoring the effect of chemotherapy in a mixed glioma by C-11-methionine PET. J Neuroimaging 2003;13:269–271
Tsuyuguchi N, Sunada I, Iwai Y, Yamanaka K, Tanaka K, Takami T, et al. Methionine positron emission tomography of recurrent metastatic brain tumor and radiation necrosis after stereotactic radiosurgery: is a differential diagnosis possible? J Neurosurg 2003;98:1056–1064
Wurker M, Herholz K, Voges J, Pietrzyk U, Treuer H, Bauer B, et al. Glucose consumption and methionine uptake in low-grade gliomas after iodine-125 brachytherapy. Eur J Nucl Med 1996;23:583–586
Lilja A, Lundqvist H, Olsson Y, Spannare B, Gullberg P, Langstrom B. Positron emission tomography and computed tomography in differential diagnosis between recurrent or residual glioma and treatment-induced brain lesions. Acta Radiol 1989;30:121–128
Popperl G, Goldbrunner R, Gildehaus FJ, Kreth FW, Tanner P, Holtmannspotter M, et al. O-(2-[18F]fluoroethyl)-l-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging 2005;32:1018–1025
Swets JA. ROC analysis applied to the evaluation of medical imaging techniques. Invest Radiol 1979;14:109–121
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982;143:29–36
Marriott CJ, Thorstad W, Akabani G, Brown MT, McLendon RE, Hanson MW, et al. Locally increased uptake of fluorine-18-fluorodeoxyglucose after intracavitary administration of iodine-131-labeled antibody for primary brain tumors. J Nucl Med 1998;39:1376–1380
Kaim AH, Weber B, Kurrer MO, Westera G, Schweitzer A, Gottschalk J, et al. 18F-FDG and 18F-FET uptake in experimental soft tissue infection. Eur J Nucl Med Mol Imaging 2002;29:648–654
Spaeth N, Wyss MT, Weber B, Scheidegger S, Lutz A, Verwey J, et al. Uptake of 18F-fluorocholine, 18F-fluoroethyl-L-tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: implications for separation of radiation necrosis from tumor recurrence. J Nucl Med 2004;45:1931–1938
Langen KJ, Muhlensiepen H, Holschbach M, Hautzel H, Jansen P, Coenen HH. Transport mechanisms of 3-[123I]iodo-alpha-methyl-L-tyrosine in a human glioma cell line: comparison with [3H]methyl]-L-methionine. J Nucl Med 2000;41:1250–1255
Miyagawa T, Oku T, Uehara H, Desai R, Beattie B, Tjuvajev J, et al. “Facilitated” amino acid transport is upregulated in brain tumors. J Cereb Blood Flow Metab 1998;18:500–509
Heiss P, Mayer S, Herz M, Wester HJ, Schwaiger M, Senekowitsch-Schmidtke R. Investigation of transport mechanism and uptake kinetics of O-(2-[18F]fluoroethyl)-L-tyrosine in vitro and in vivo. J Nucl Med 1999;40:1367–1373
Rachinger W, Goetz C, Popperl G, Gildehaus FJ, Kreth FW, Holtmannspotter M, et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery 2005;57:505–511
Acknowledgement
Part of this work was supported by grants from the Deutsche Forschungsgemeinschaft [Forschergruppe Radionuklidtherapie (FOR 411) GO 998/1-1 (2)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pöpperl, G., Götz, C., Rachinger, W. et al. Serial O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of intracavitary radioimmunotherapy in patients with malignant glioma. Eur J Nucl Med Mol Imaging 33, 792–800 (2006). https://doi.org/10.1007/s00259-005-0053-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0053-7