Abstract
Purpose
Previous studies suggest that radiolabelled amino acids could be superior to FDG in differentiating tumour and inflammation. Therefore the aim of this study was to investigate the uptake of FET and MET in human tumour and inflammatory cells and to investigate their uptake kinetics.
Methods
For uptake studies, cells were incubated with 370 kBq FET or 3.7 kBq MET for 15 min. Kinetic studies were performed at variable concentrations of FET and MET. Competitive inhibition studies were done with BCH, MeAIB and L-serine.
Results
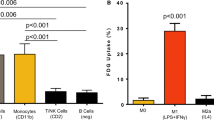
All inflammatory cells incorporated more MET than the tumour cells. The uptake of FET, in contrast, was significantly lower in all inflammatory cells than in the tumour cells. In tumour cells the uptake of MET was about five times the uptake of FET. The competitive inhibitors reduced uptake of both tracers to 20–40% in tumour cells and to 70% in inflammatory cells. Kinetic studies showed that MET and FET transport was saturable in all cells except macrophages and followed a Michaelis-Menten kinetic. Highest capacity (V max) and affinity (K m) for the uptake of MET was observed in granulocytes. Capacity and affinity for FET uptake were highest in the DHL-4 cells.
Conclusion
In contrast to MET, FET accumulated to a significantly greater extent in tumour cells than in inflammatory cells. The marked differences between tumour and inflammatory cells concerning FET and MET uptake suggest that FET and MET are substrates of different subtypes of the L system.




Similar content being viewed by others
References
Larson SM. Cancer or inflammation? A holy grail for nuclear medicine. J Nucl Med 1994;35:1653–1654
Bakheet SM, Powe J. Benign causes of 18-FDG uptake on whole body imaging. Semin Nucl Med 1998;28:352–358
Kubota R, Yamada S, Kubota K, Ishiwata K, Tamahashi N, Ido T. Intratumoral distribution of fluorine-18-fluordeoxyglucose in vivo: high accumulation in macrophages and granulation tissues studied by microautoradiography. J Nucl Med 1992;33:1972–1980
Kubota K, Kubota R, Yamada S, Tada M. Effects of radiotherapy on the cellular uptake of carbon-14-labeledL-methionine in tumor tissue. Nucl Med Biol 1995;22:193–198
Kubota R, Kubota K, Yamada S, Tada M, Iwata R, Tamahashi N. Methionine uptake by tumor tissue: a microautoradiographic comparison with FDG. J Nucl Med 1995;36:484–492
Yamada S, Kubota K, Kubota R, Ido T, Tamahashi N. High accumulation in fluorine-18-fluorodeoxyglucose in turpentine-induced inflammatory tissue. J Nucl Med 1995;36:1301–1306
Sugawara Y, Gutowski TD, Fisher SJ, Brown R, Wahl RL. Uptake of positron emission tomography tracers in experimental bacterial infections: a comparative biodistribution study of radiolabeled FDG, thymidine andL-methionine,67Ga-citrate and125I-HAS. Eur J Nucl Med 1999;26:333–341
Reinhardt MJ, Kubota K, Yamada S, Iwata R, Yaegashi H. Assessment of cancer recurrence in residual tumors after fractionated radiotherapy: a comparison of fluordeoxyglucose,L-methionine and thymidine. J Nucl Med 1997;38:280–287
Gutowski TD, Fisher SJ, Moon R, Wahl RL. Experimental studies of18F-2-fluoro-2-deoxy-D-glucose (FDG) in infection and in reactive lymph nodes [abstract]. J Nucl Med 1992;33:925
Wahl RL, Fisher SJ. A comparison of FDG,L-methionine and thymidine accumulation into experimental infections and reactive lymph nodes [abstract]. J Nucl Med 1993;34:104
Heiss P, Mayer S, Herz M, Wester HJ, Schwaiger M, Senekowitsch-Schmidtke R. Investigation of transport mechanism and uptake kinetics ofO-(2-[18F]fluoroethyl)-L-tyrosine in vitro and in vivo. J Nucl Med 1999;40:1367–1373
Laverman P, Boerman OC, Corstens FH, Oyen WJ. Fluorinated amino acids for tumour imaging with positron emission tomography. Eur J Nucl Med Mol Imaging 2002;29:681–690
Jager PL, Vaalburg W, Pruim J, de Vries EG, Langen KJ, Piers DA. Radiolabeled amino acids: basic aspects and clinical applications in oncology. J Nucl Med 2001;42:432–445
Samnick S, Hellwig D, Gouverneur E, Romeike BF, Schneider G, Menger M, et al Evaluation of radioiodinated phenylalanine-analogues as radiopharmaceuticals to image pancreatic carcinomas in in-vivo models of human pancratic tumors. Eur J Nucl Med Mol Imaging 2003;30 Suppl 2:S175
Langen KJ, Ziemons K, Kiwit CW, Herzog H, Kuwert T, Bock WJ, et al 3-[I-123]iodo-α-methyltyrosine and [methyl-C-11]-L-methionine uptake in cerebral gliomas: a comparative study using SPECT and PET. J Nucl Med 1997;38:517–522
Leskinen-Kallio S, Ruotsalainen U, Nagren K, Teras M, Joensuu H. Uptake of carbon-11-methionine and fluorodeoxyglucose in non-Hodgkin’s lymphoma: a PET study. J Nucl Med 1991;32:1211–1218
Nettelbladt OS, Sundin AE, Valind SO, Gustafsson GR, Lamberg K, Lagström B, et al Combined fluorine-18-FDG and carbon-11-methionine PET for diagnosis of tumors in lung and mediastinum. J Nucl Med 1998;39:640–647
Leskinen-Kallio S, Nagren K, Lehikoinen P, Ruotsalainen U, Joensuu H. Uptake of C-11-methionine in breast cancer studied by PET: an association with the size of S-phase fraction. Br J Cancer 1991;64:1121–2214
Kole AC, Plaat BE, Hoekstra HJ, Vaalburg W, Molenaar WM. FDG andL-[1-11C]-tyrosine imaging of soft-tissue tumors before and after therapy. J Nucl Med 1999;40:381–386
Tomiyoshi K, Inoue T, Higuchi T, Ahmed K, Sarwar M, Alyafei S, et al Metabolic studies of [F-18-alpha-methyl]tyrosine in mice bearing colorectal carcinoma LS-180. Anitcancer Drugs 1999;10:329–336
Langen KJ, Jarosch M, Hamacher K, Muhlensiepen H, Weber F, Floeth F, Pauleit D, Herzog H, Coenen HH. Imaging of gliomas with Cis-4-[18F]fluoro-L-proline. Nucl Med Biol 2004;31:67–75
Langen KJ, Jarosch M, Mühlensiepen H, Hamacher K, Broer S, Jansen P, et al Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl Med Biol 2003;30:501–508
Wester HJ, Herz M, Weber W, Heiss P, Senekowitsch-Schmidtke R, Schwaiger M, et al Synthesis and radiopharmacology ofO-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med 1999;40:205–212
Weber WA, Wester HJ, Grosu AL, Herz M, Dzewas B, Feldmann HJ, et alO-(2-[18F]fluoroethyl)-L-tyrosine andL-[methyl-11C]methionine uptake in brain tumors: initial results of a comparative study. Eur J Nucl Med Mol Imaging 2000;27:542–549
Rau FC, Weber WA, Wester HJ, Herz M, Becker I, Krüger A, et al O-(2-[18F]Fluoroethyl)-L-tyrosine (FET): a tracer for diferentiation of tumour from inflammation in murine lymph nodes. Eur J Nucl Med Mol Imaging 2002;29:1039–1046
Pöpperl G, Götz C, Rachinger W, Gildehaus FJ, Tonn JC, Tatsch K. Value ofO-(2-[18F]fluoroethyl)-L-tyrosine PET for the diagnosis of recurrent glioma. Eur J Nucl Med Mol Imaging 2004;31:1464–1470
Weckesser M, Langen KJ, Rickert CH, Kloska S, Straeter R, Hamacher K, et alO-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging 2005;32:422–429
Kaim AH, Weber B, Kurrer MO, Westera G, Schweitzer A, Gottschalk J, et al 18F-FDG and 18F-FET uptake in experimental soft tissue infection. Eur J Nucl Med Mol Imaging 2002;29:648–654
Pauleit D, Floeth F, Tellmann L, Hamacher K, Hautzel H, Müller HW, et al Comparison ofO-(2-18F-fluoroethyl)-L-tyrosine PET and 3-123I-iodo alpha-methyl-L-tyrosine SPECT in brain tumors. J Nucl Med 2004;45:374–381
Ishiwata K, Kubota K, Murakami M, Kubota R, Sasaki T, Ishii S, et al Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J Nucl Med 1993;34:1936–1943
Tahara T, Ichiya Y, Kuwabara Y. High fluorodeoxyglucose uptake in abdominal abscesses: a PET study. J Comput Assist Tomog 1989;13:829–831
Meyer MA, Frey KA, Schwaiger M. Discordance between F-18 fluordeoxyglucose uptake and contrast enhancement in a brain abscess. Clin Nucl Med 1993;18:682–684
Kubota K, Matsuzawa T, Fumiwata T. Differential diagnosis of lung tumor with positron emission tomography: a prospective study. J Nucl Med 1990;31:1927–1933
Lowe VJ, Naunheim KS. Positron emission tomography in lung cancer. Ann Thorac Surg 1998;65:1821–1829
Suguwara Y, Braun DK, Kison PV, Russo JE, Zasadny KR, Wahl RL. Rapid detection of human infections with fluorine-18-fluorodeoxyglucose and positron emission tomography: preliminary results. Eur J Nucl Med 1998;25:1238–1243
Guhlmann A, Brecht-Krauss D, Suger G, Glatting G, Kotzerke J, Kinzl L, et al Fluorine-18-FDG PET and technetium-99m antigranulocyte antibody scintigraphy in chronic osteomyelitis. J Nucl Med 1998;39:2145–2152
Spaeth N, Wyss MT, Weber B, Scheidegger S, Lutz A, Verwey J, et al Uptake of 18F-fluorocholine, 18F-fluoroethyl-L-tyrosine, and 18F-FDG in acute cerebral radiation injury in the rat: implications for separation of radiation necrosis from tumor recurrence. J Nucl Med 2004;45:1931–1938
Pauleit D, Stoffels G, Schaden W, Hamacher K, Bauer D, Tellmann L, et al PET withO-(2-18F-fluoroethyl)-L-tyrosine in peripheral tumors: first clinical results. J Nucl Med 2005;46:411–416
Kubota K, Matsuzawa T, Fujiwara T, Sato T, Tada M, Ido T, Ishiwata K. Differential diagnosis of AH 109 A tumor and inflammation by radioscintigraphy withL-[methyl-11C]methionine. Jpn J Cancer Res 1989;80:778–782
Pauleit D, Floeth F, Herzog H, Hamacher K, Tellmann L, Müller HW, Coenen HH, Langen KJ. Whole-body distribution and dosimety ofO-(2-[18F]fluoroethyl)-L-tyrosine. Eur J Nucl Med Mol Imaging 2003;30:519–524
Shotwell MA, Kilberg MS, Oxender DL. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta 1983;737:267–284
Saier MH, Daniels GA, Boerner P, Lin J. Neutral amino acid transport systems in animal cells: potential targets of oncogene action and regulators of cellular growth. J Membr Biol 1988;104:1–20
Segel GB, Woodlock TJ, Murant FG, Lichtman MA. Photoinhibition of 2-amino-2-carboxybicyclo[2,2,1]heptane transport byO-diazoacetyl-L-serine. An initial step in identifying the L-system amino acid transporter. J Biol Chem 1989;264:16399–402
Barker GA, Wilkins RJ, Golding S, Ellory JC. Neutral amino acid transport in bovine articular chondrocytes. J Physiol 1999;514:795–808
Jara JR, Martinez-Liarte JH, Solano F, Penafiel R. Transport ofL-tyrosine by B16/F10 melanoma cells: the effect of the intracellular content of other amino acids. J Cell Sci 1990;97:479–485
Pankovich JM, Jimbow K. Tyrosine transport in a human melanoma cell line as a basis for selective transport of cytotoxic analogues. Biochem J 1991;280:721–725
Langen KJ, Pauleit D, Coenen HH. 3-[123I]Iodo-alpha-methyl-L-tyrosine: uptake mechanisms and clinical applications. Nucl Med Biol 2002;29:625–631
Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch 2003;445:529–533
Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990;70:43–77
Scanion K, Berkowitz K, Pallai M, Waxman S. Inhibition of methionine transport by methotrexate in mitogen stimulated human lymphocytes. Cancer Treat Rep 1983;67:631–639
Rau FC, Philippi H, Rubio-Aliaga I, Daniel H, Weber A, Schwaiger M, et al Identification of subtypes of amino acid transporters in human tumor and inflammatory cells by reverse transcription-polymerase chain reaction [abstract]. J Nucl Med 2002;43(suppl):24
Verrey F, Meier C, Rossier G, Kuehn LC. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Eur J Physiol 2000;440:503–512
Su TZ, Lunney E, Campbell G, Oxender DL. Transport of gabapentin, a gamma-amino acid drug, by system L alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes and CHO cells. J Neurochem 1995;64:2125–2131
Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, et al Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 2001;1514:291–302
Shennan DB, Thomson J, Barber MC, Travers MT. Functional and molecular characteristics of system L in human breast cancer cells. Biochim Biophys Acta 2003;1611:81–90
Ohkame H, Masuda H, Ishii Y, Kanai Y. Expression of L-type amino acid transporter 1 (LAT1) and 4F2 heavy chain (4F2hc) in liver tumor lesions of rat models. J Surg Oncol 2001;78:265–271
Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J 2002;21:580–589
Lahoutte T, Caveliers v, Camargo S, Franca R, Ramadan T, Veljkovic E, Mertens J, et al SPECT and PET amino acid tracer influx via system L (h4F2hc-hLAT1) and its transstimulation. J Nucl Med 2004;45:1591–1596
Babu E, Kanai Y, Chairoungdua A, Kim do K, Iribe Y, Tangtrongsup S, et al Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem 2003;278:43838–43845
Collarini EJ, Campbell GS, Oxender DL. Evidence for a regulatory element controlling amino acid transport system L in Chinese hamster ovary cells. J Cell Biochem 1994;56:544–549
Shotwell MA, Jayme DW, Kilberg MS, Oxender DL. Neutral amino acid transport system in Chinese hamster ovary cells. J Biol Chem 1981;256:5422–5427
Mitsumoto Y, Sato K, Ohyashiki T, Mohri T. Leucine-proton cotransport system in Chang liver cell. J Biol Chem 1983;261:4549–4554
Rajan DP, Kekuda R, Huang W, Devoe LD, Leibach FH, Prasad PD, et al Cloning and functional characterization of a Na+-independent, broad-specific neutral amino acid transporter from mammalian intestine. Biochim Biophys Acta 2001;463:6–14
Acknowledgements
Useful advice given by P. Heiss, F. Rau and H. Philippi is gratefully acknowledged, as is the support provided by H. Hochrein, Institut für Mikrobiologie, Immunologie und Hygiene der Technischen Universität München, Munich, Germany.
Author information
Authors and Affiliations
Corresponding author
Additional information
Barbara Stöber and Ursula Tanase contributed equally to this work.
Rights and permissions
About this article
Cite this article
Stöber, B., Tanase, U., Herz, M. et al. Differentiation of tumour and inflammation: characterisation of [methyl-3H]methionine (MET) and O-(2-[18F]fluoroethyl)-L-tyrosine (FET) uptake in human tumour and inflammatory cells. Eur J Nucl Med Mol Imaging 33, 932–939 (2006). https://doi.org/10.1007/s00259-005-0047-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0047-5