Abstract
Purpose
SPECT examinations of neurotransmitter systems in the brain have to be comparable between centres to generate a comprehensive data pool, e.g. for multicentre studies. Equipment-specific effects on quantitative evaluations and corresponding methods for compensation, however, have been insufficiently examined. Previous studies have shown that quantitative results may vary significantly according to the imaging equipment used, thereby affecting clinical interpretation of the data. The aim of this study was to determine correction factors for common camera/collimator combinations based on standardised measurements of an anthropomorphic 3D basal ganglia phantom to compensate for the effects of different SPECT camera/collimator equipment. The latter may serve as a model for human studies of the dopaminergic system.
Methods
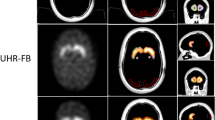
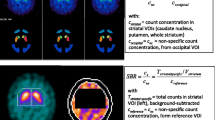
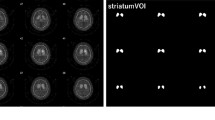
The striatum and background chambers of a commercially available phantom (RSD Alderson) were filled with various 123I concentrations encompassing specific striatum/background ratios from 0.6 to 16.1. This setup was imaged with the following four camera/collimator combinations: Siemens Multispect 3 fitted with LEHR and 123I parallel-hole collimators, Siemens ECAM with LEHR parallel-hole collimators and Philips Prism 3000 fitted with LEHR fanbeam collimators, using standardised protocols for acquisition and reconstruction. All scans were automatically co-registered to a SPECT template of the phantom and quantified using a 3D volume of interest (VOI) map based on a CT scan of the phantom. All striatal/background ratios calculated by SPECT were compared with the true ratios calculated from the measurements in a well counter. Regression analyses were performed and recovery correction factors between measured and true ratios determined.
Results
The relation between true and measured ratios could be sufficiently described by a linear regression for each camera/collimator combination without relevant improvement when using second-order polynomial regression models. The recovery correction factors and standard errors were 2.04±0.04 for the Philips Prism 3000, 2.67±0.03 for the Siemens Multispect 3/LEHR parallel-hole collimators, 2.15±0.03 for the Siemens Multispect 3/123I collimators and 2.81±0.03 for the Siemens ECAM. Percentage recovery ranged from 36% to 49%.
Conclusion
Measurements of a 3D basal ganglia phantom with various imaging devices revealed linear correlations between measured and true striatal/background ratios. Based on these findings, adjustment of quantitative results between different equipment seems possible, provided that acquisition, reconstruction and evaluation are adequately standardised. The use of identical evaluation methods in phantom and patient studies (comparable shape, size and location of the VOIs) might allow transfer of the calculated correction factors from phantom to studies of the dopaminergic system in patients.



Similar content being viewed by others
References
Bergmann H, Busemann-Sokole E, Horton PW. Quality assurance and harmonisation of nuclear medicine investigations in Europe. Eur J Nucl Med 1995;22:477–480
Meyer PT, Sattler B, Lincke T, Seese A, Sabri O. Investigating dopaminergic neurotransmission with 123I-FP-CIT SPECT: comparability of modern SPECT systems. J Nucl Med 2003;44:839–845
Stodilka RZ, Kemp BJ, Prato FS, Nicholson RL. Importance of bone attenuation in brain SPECT quantification. J Nucl Med 1998;39:190–197
Tatsch K, Asenbaum S, Bartenstein P, Catafau A, Halldin C, Pilowsky LS, et al European Association of Nuclear Medicine procedure guidelines for brain neurotransmission SPET using 123I-labelled dopamine D2 transporter ligands. Eur J Nucl Med Mol Imaging 2002;29:BP30–BP35
Radau PE, Linke R, Slomka PJ, Tatsch K. Optimization of automated quantification of 123I-IBZM uptake in the striatum applied to parkinsonism. J Nucl Med 2000;41:220–227
Radau PE, Koch W, Holtmannspoetter M, Poepperl G, Tatsch K. Automated, objective software for 3D registration and analysis of dopamine transporter SPECT studies. J Nucl Med 2003;44:P12
Koch W, Radau PE, Hamann C, Tatsch K. Clinical testing of an optimized software solution for an automated, observer-independent evaluation of dopamine transporter SPECT studies. J Nucl Med 2005;46:1109–1118
Koch W, Münzing W, Geworski L, Sonnenschein W, Höffken H, Czech N, et al Zentrumübergreifende Messungen an einem Basalganglienphantom zur Vereinheitlichung der SPECT Diagnostik des dopaminergen Systems. In: Brink I, editor. Nuklearmedizin als Paradigma molekularer Bildgebung. Berlin, Germany: Blackwell; 2002. p. 20
Hudson H, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994;13:594–600
Kauppinen T, Yang J, Kilpelainen H, Kuikka JT. Quantitation of neuroreceptors: a need for better SPECT imaging. Nuklearmedizin 2001;40:102–106
Booij J, Hemelaar TG, Speelman JD, de Bruin K, Janssen AG, van Royen EA. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J Nucl Med 1999;40:753–761
Pirker W, Asenbaum S, Hauk M, Kandlhofer S, Tauscher J, Willeit M, et al Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Nucl Med 2000;41:36–44
Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I, et al SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. Imaging serotonin and dopamine transporters with 123I-beta-CIT SPECT: binding kinetics and effects of normal aging. J Neural Transm Gen Sect 1993;94:137–146
Geworski L, Knoop BO, de Cabrejas ML, Knapp WH, Munz DL. Recovery correction for quantitation in emission tomography: a feasibility study. Eur J Nucl Med 2000;27:161–169
Tatsch K, Asenbaum S, Bartenstein P, Catafau A, Halldin C, Pilowsky LS, et al European Association of Nuclear medicine procedure guidelines for brain neurotransmission SPET using 123I-labelled dopamine D2 receptor ligands. Eur J Nucl Med Mol Imaging 2002;29:BP23–BP29
Hamann C, Koch W, Radau PE, Tatsch K. Iterative reconstruction or filtered backprojection for quantitative assessment of dopamine d2 receptor studies? J Nucl Med 2003;44:114P
Hashimoto J, Kubo A, Ogawa K, Amano T, Fukuuchi Y, Motomura N, et al Scatter and attenuation correction in technetium-99m brain SPECT. J Nucl Med 1997;38:157–162
Hashimoto J, Sasaki T, Ogawa K, Kubo A, Motomura N, Ichihara T, et al Effects of scatter and attenuation correction on quantitative analysis of beta-CIT brain SPET. Nucl Med Commun 1999;20:159–165
Soret M, Koulibaly PM, Darcourt J, Hapdey S, Buvat I. Quantitative accuracy of dopaminergic neurotransmission imaging with 123I SPECT. J Nucl Med 2003;44:1184–1193
Yang J, Kuikka JT, Vanninen E, Kauppinen T, Lansimies E, Patomaki L. Evaluation of scatter correction using a single isotope for simultaneous emission and transmission data. Phantom and clinical patient studies. Nuklearmedizin 1999;38:49–55
Kauppinen T, Koskinen MO, Alenius S, Vanninen E, Kuikka JT. Improvement of brain perfusion SPET using iterative reconstruction with scatter and non-uniform attenuation correction. Eur J Nucl Med 2000;27:1380–1386
Ichihara T, Ogawa K, Motomura N, Kubo A, Hashimoto S. Compton scatter compensation using the triple-energy window method for single- and dual-isotope SPECT. J Nucl Med 1993;34:2216–2221
El Fakhri G, Buvat I, Benali H, Todd-Pokropek A, Di Paola R. Relative impact of scatter, collimator response, attenuation, and finite spatial resolution corrections in cardiac SPECT. J Nucl Med 2000;41:1400–1408
Yanch JC, Flower MA, Webb S. Improved quantification of radionuclide uptake using deconvolution and windowed subtraction techniques for scatter compensation in single photon emission computed tomography. Med Phys 1990;17:1011–1022
Jaszczak RJ, Greer KL, Floyd CE Jr, Harris CC, Coleman RE. Improved SPECT quantification using compensation for scattered photons. J Nucl Med 1984;25:893–900
El Fakhri G, Moore SC, Maksud P, Aurengo A, Kijewski MF. Absolute activity quantitation in simultaneous 123I/99mTc brain SPECT. J Nucl Med 2001;42:300–308
Linke R, Gostomzyk J, Hahn K, Tatsch K. [123I]IPT binding to the presynaptic dopamine transporter: variation of intra- and interobserver data evaluation in parkinsonian patients and controls. Eur J Nucl Med 2000;27:1809–1812
Hamann C, Koch W, Radau PE, Tatsch K. Automated evaluation of dopamine D2 receptor SPECT studies: Benefit in clinical routine? J Nucl Med 2003;44:P261–P262
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koch, W., Radau, P.E., Münzing, W. et al. Cross-camera comparison of SPECT measurements of a 3-D anthropomorphic basal ganglia phantom. Eur J Nucl Med Mol Imaging 33, 495–502 (2006). https://doi.org/10.1007/s00259-005-0036-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0036-8