Abstract
Purpose
The purpose of this study was to design a fully automatic computer-assisted diagnostic system for early- and late-onset mild Alzheimer’s disease (AD).
Methods
Glucose metabolic images were obtained from mild AD patients and normal controls using positron emission tomography (PET) and 18F-fluorodeoxyglucose (FDG). Two groups of 20 mild AD patients with different ages of onset were examined. A fully automatic diagnostic system using the statistical brain mapping method was established from the early-onset (EO) and late-onset (LO) groups, with mean ages of 59.1 and 70.9 years and mean MMSE scores of 23.3 and 22.8, respectively. Aged-matched normal subjects were used as controls. We compared the diagnostic performance of visual inspection of conventional axial FDG PET images by experts and beginners with that of our fully automatic diagnostic system in another 15 EO and 15 LO AD patients (mean age 58.4 and 71.7, mean MMSE 23.6 and 23.1, respectively) and 30 age-matched normal controls. A receiver operating characteristic (ROC) analysis was performed to compare data.
Results
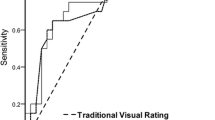
The diagnostic performance of the automatic diagnostic system was comparable with that of visual inspection by experts. The area under the ROC curve for the automatic diagnostic system was 0.967 for EO AD patients and 0.878 for LO AD patients. The mean area under the ROC curve for visual inspection by experts was 0.863 and 0.881 for the EO and LO AD patients, respectively. The mean area under the ROC curve for visual inspection by beginners was 0.828 and 0.717, respectively.
Conclusion
The fully automatic diagnostic system for EO and LO AD was able to perform at a similar diagnostic level to visual inspection of conventional axial images by experts.






Similar content being viewed by others
References
Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med 2001;15:85–92
Ishii K. Clinical application of positron emission tomography for diagnosis of dementia. Ann Nucl Med 2002;16:515–25
Poulin P, Zakzanis KK. In vivo neuroanatomy of Alzheimer’s disease: evidence from structural and functional brain imaging. Brain Cogn 2002;49:220–5
Patwardhan MB, McCrory DC, Matchar DB, Samsa GP, Rutschmann OT. Alzheimer disease: operating characteristics of PET—a meta-analysis. Radiology 2004;231:73–80
Minoshima S. Imaging Alzheimer’s disease: clinical applications. Neuroimaging Clin North Am 2003;13:769–80
Herholz K. PET studies in dementia. Ann Nucl Med 2003;17:79–89
Van Heertum RL, Tikofsky RS. Positron emission tomography and single-photon emission computed tomography brain imaging in the evaluation of dementia. Semin Nucl Med 2003;33:77–85
Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann Neurol 1997;42:85–94
Ishii K, Sasaki M, Matsui M, Sakamoto S, Yamaji S, Hayashi N, et al. A diagnostic method for suspected Alzheimer’s disease using H2 15O positron emission tomography perfusion Z score. Neuroradiology 2000;42:787–94
Iversen LL. Differences between early and late-onset Alzheimer’s disease. Neurobiol Aging 1987;8:554–5
Ishii K, Kawachi T, Sasaki H, Kono AK, Fukuda T, Kojima Y, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer’s disease and assessment of diagnostic performance of Z score images. AJNR Am J Neuroradiol 2005;25:1–8
Mielke R, Herholz K, Grond M, Kessler J, Heiss WD. Differences of regional cerebral glucose metabolism between presenile and senile dementia of Alzheimer type. Neurobiol Aging 1992;13:93–8
Yasuno F, Imamura T, Hirono N, Ishii K, Sasaki M, Ikejiri Y, et al. Age at onset and regional cerebral glucose metabolism in Alzheimer’s disease. Dement Geriatr Cogn Disord 1998;9:63–7
Sakamoto S, Ishii K, Sasaki M, Hosaka K, Mori T, Matsui M, et al. Differences in cerebral metabolic impairment between early and late onset types of Alzheimer’s disease. J Neurol Sci 2002;200:27–32
Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med 1995;36:1238–48
Burdette JH, Minoshima S, Vander Borght T, Tran DD, Kuhl DE. Alzheimer disease: Improved visual interpretation of PET images by using three-dimensional stereotaxic surface projections. Radiology 1996;198:837–43
Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, et al. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage 2002;17:302–16
Ishii K, Willoch F, Minoshima S, Drzezga A, Ficaro EP, Cross DJ, et al. Statistical brain mapping of 18F-FDG PET in Alzheimer’s disease: validation of anatomic standardization for atrophied brains. J Nucl Med 2001;42:548–57
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–44
Ishii K, Sasaki M, Kitagaki H, Yamaji S, Sakamoto S, Matsuda K, et al. Reduction of cerebellar glucose metabolism in advanced Alzheimer’s disease. J Nucl Med 1997;38:925–8
Ishii K, Yamaji S, Mori E. Cerebellar metabolic reduction in Alzheimer’s disease and data normalization. J Nucl Med 1998;39:375–6
Imabayashi E, Matsuda H, Asada T, Ohnishi T, Sakamoto S, Nakano S, et al. Superiority of 3-dimensional stereotactic surface projection analysis over visual inspection in discrimination of patients with very early Alzheimer’s disease from controls using brain perfusion SPECT. J Nucl Med 2004;45:1450–7
Minoshima S, Frey KA, Foster NL, Kuhl DE. Preserved pontine glucose metabolism in Alzheimer disease: a reference region for functional brain image (PET) analysis. J Comput Assist Tomogr 1995;19:541–7
Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology 1998;51:125–30
Ishii K, Sakamoto S, Sasaki M, Kitagaki H, Yamaji S, Hashimoto M, et al. Cerebral glucose metabolism in patients with frontotemporal dementia. J Nucl Med 1998;39:1875–8
Hosaka K, Ishii K, Sakamoto S, Mori T, Sasaki M, Hirono N, et al. Voxel-based comparison of regional cerebral glucose metabolism between PSP and corticobasal degeneration. J Neurol Sci 2002;199:67–71
Hirono N, Ishii K, Sasaki M, Kitagaki H, Hashimoto M, Imamura T, et al. Features of regional cerebral glucose metabolism abnormality in corticobasal degeneration. Dement Geriatr Cogn Disord 2000;11:139–46
Acknowledgement
The authors thank Mr. Shuya Miki (Nihon Medi-Physics, Nishinomiya, Hyogo, Japan) for providing and improving the iSSP program which uses the 3D-SSP program and was dedicated to this automatic method.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ishii, K., Kono, A.K., Sasaki, H. et al. Fully automatic diagnostic system for early- and late-onset mild Alzheimer’s disease using FDG PET and 3D-SSP. Eur J Nucl Med Mol Imaging 33, 575–583 (2006). https://doi.org/10.1007/s00259-005-0015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-005-0015-0