Abstract
Purpose
Cu-diacetyl-bis(N4-methylthiosemicarbazone (Cu-ATSM) is an effective marker for the delineation of hypoxic tissue. Dosimetry calculations by the established Medical Internal Radionuclide Dose (MIRD) approach were performed with both animal and patient data.
Methods
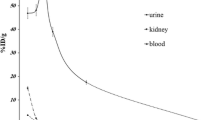
Human absorbed dose estimates extrapolated from rat data were based on the biodistribution of 61Cu-ATSM in adult rats. Eighteen tissues were harvested and time–activity curves generated. The measured residence times and the MIRD S-values for 60Cu-ATSM were used to estimate human absorbed doses. The biodistribution of the tracer was directly measured in five patients injected with approximately 480 MBq of 60Cu-ATSM and imaged by positron emission tomography (PET) with a whole-body protocol. The combined data from all patients were used to derive organ residence times, and organ doses were calculated by MIRD methodology for 60Cu-ATSM, 61Cu-ATSM, 62Cu-ATSM, and 64Cu-ATSM.
Results
Human absorbed dose estimates extrapolated from rat biodistribution data indicated that the kidneys appeared to be the dose-limiting organ (0.083 mGy/MBq) with a whole-body dose of 0.009 mGy/MBq. Based on the human PET imaging data, the liver appeared as the dose-limiting organ, with an average radiation dose of 0.064 mGy/MBq. The whole-body dose was 0.009 mGy/MBq and the effective dose was 0.011 mSv/MBq.
Conclusion
These relatively small absorbed doses to normal organs allow for the safe injection of 500–800 MBq of 60Cu-ATSM, which is sufficient for PET imaging in clinical trials.



Similar content being viewed by others
References
Crabtree HG, Cramer W. The action of radium on cancer cells I and II. Some factors determining the susceptibility of cancer cells to radium. Proc R Soc Ser B 1933;113:238–50.
Brown JM. The hypoxic cell: A target for selective cancer therapy—Eighteenth Bruce F. Cain Memorial Award Lecture. Cancer Res 1999;59:5863–70.
Gray LH, Conger AD, Ebert M, Hornsey S, Scott OC. Concentration of oxygen dissolved in tissues at the time of irradiation as a factor in radiotherapy. Br J Radiol 1953;26:638–48.
Tannock I, Guttman P. Responses of Chinese hamster ovary cells to anticancer drugs under aerobic and hypoxic conditions. Br J Cancer 1981;42:245–8.
Lewis JS, Welch MJ. PET Imaging of hypoxia. Q J Nucl Med 2001;45:183–8.
Koh W-J, Rasey JS, Evans ML. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992;22:199–212.
Rasey JS, Koh W-J, Evans ML, Peterson LM, Lewellen TK, Graham MM, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys 1996;36:417–28.
Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 1997;38:1155–60.
Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of 64Cu-ATSM in vivo and in vitro in a hypoxic tumor model. J Nucl Med 1999;40:177–83.
Lewis JS, Sharp TL, Laforest R, Fujibayashi Y, Welch MJ. Tumor uptake of copper-diacetyl-bis(N4-methylthiosemicarbazone): effect of changes in tissue oxygenation. J Nucl Med 2001;42:655–61.
Dehdashti F, Mintun MA, Lewis JS, Bradley J, Govindan R, Laforest R, et al. In vivo assesment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging 2003;30:844–50.
Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response—a preliminary report. Int J Radiat Oncol Biol Phys 2003;55:1233–8.
Haynes NG, Lacy JL, Nayak N, Martin CS, Dai D, Mathias CJ, et al. Performance of a 62Zn/62Cu generator in clinical trials of PET perfusion agent 62Cu-PTSM. J Nucl Med 2000;41:309–14.
Fujibayashi Y, Matsumoto K, Yonekura Y, Konishi J, Yokoyama A. A new zinc-62/copper-62 generator as a copper-62 source for PET radiopharmaceuticals. J Nucl Med 1989;30:1838–42.
McCarthy DW, Shefer RE, Klinkowstein RE, Bass LA, Margeneau WH, Cutler CS, et al. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl Med Biol 1997;24:35–43.
McCarthy DW, Bass LA, Cutler PD, Shefer RE, Klinkowstein RE, Herrero P, et al. High purity production and potential applications of copper-60 and copper-61. Nucl Med Biol 1999;26:351–8.
National Nuclear Data Center, BrookHaven National Laboratory, http://www.nndc.bnl.gov/mird
Anderson CJ, Jones LA, Bass LA, Sherman EL, McCarthy DW, Cutler PD, et al. Radiotherapy, toxicity and dosimetry of copper-64-TETA-octreotide in tumor bearing rats. J Nucl Med 1998;39:1944–51.
Lewis JS, Lewis MR, Cutler PD, Srinivasan A, Schmidt MA, Schwarz SW, et al. Radiotherapy and dosimetry of 64Cu-TETA-Tyr3-octreotate in a somatostatin receptor-positive, tumor bearing rat model. Clin Cancer Res 1999;5:3608–16.
Lewis JS, Laforest R, Lewis MR, Anderson CJ. Comparative dosimetry of copper-64 and yttrium-90-labeled somatostatin analogs in a tumor-bearing rat model. Cancer Biotherapy Radiopharm 2000;15:593–604.
Stabin MG. MIRDOSE: Personal computer software for internal dose assessment in nuclear medicine. J Nucl Med 1996;37:538–46.
Cutler PD, Schwarz SW, Anderson CJ, Connett JM, Welch MJ, Philpott GW, et al. Dosimetry of copper-64-labeled monoclonal antibody 1A3 as determined by PET imaging of the torso. J Nucl Med 1995;36:2363–71.
Recommendations of the International Commission on Radiological Protection, 1990.
Takahashi N, Fujibayashi Y, Yonekura Y, Welch MJ, Waki A, Tsuchida T, et al. Evaluation of 62Cu labeled diacetyl-bis(N4-methylthiosemicarbazone) as a hypoxic tissue tracer in patients with lung cancer. Ann Nucl Med 2000;14:323–8.
Fritzberg AR, Bloedow DC. Animal models in the study of hepatobiliary radiotracers. In: Lambrecht RM, Eckelman WC, editors. Animal models in radiotracer design. Berlin Heidelberg New York: Springer; 1983. p. 179–209.
Acknowledgements
We thank Margaret Morris and Elizabeth Conrad for their technical assistance with the animal studies. We thank Helen Kaemmerer, Jennifer Frye, and Linda Becker for their assistance with the clinical studies. We are very grateful to Dr. Barry Siegel for editing and for providing a critical review of the manuscript. We would also like to thank Michael Stabin for providing the 60Cu and 61Cu S-value tables as those were not calculated by the standard release of MIRDOSE 3.0.
This work was supported by a National Institute of Health grant (CA81525) and by a grant from the United States Department of Energy (DE-FG02-87ER60512). Production of Cu radionuclides was partially supported by NCI Grant No. R24 CA 86307. The authors are in full control of the data and therefore agree to allow EJNM to review the data if so requested by EJNM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laforest, R., Dehdashti, F., Lewis, J.S. et al. Dosimetry of 60/61/62/64Cu-ATSM: a hypoxia imaging agent for PET. Eur J Nucl Med Mol Imaging 32, 764–770 (2005). https://doi.org/10.1007/s00259-004-1756-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1756-x