Abstract
Purpose
Myocardial glucose utilization (MGU) is altered in various heart diseases. The aim of this study was to quantitatively assess regional myocardial glucose utilization in patients with left ventricular (LV) dysfunction by dynamic 18F-fluorodeoxyglucose positron emission tomography (FDG PET).
Methods
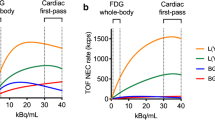
A total of 18 subjects were studied, including ten with LV dysfunction (seven with idiopathic dilated cardiomyopathy and three with aortic regurgitation; NYHA II in 8 and III in 2) and eight healthy normal volunteers. Patients with diabetes mellitus were excluded. A dynamic PET study was performed for 40 min following the injection of 370 MBq of FDG after 50-g glucose loading. On the basis of a three-compartment model, MGU, K1, k2, and k3 were computed on a pixel by pixel basis to generate LV myocardial parametric maps. FDG standardized uptake value (SUV) was also calculated using static images obtained 40 min after FDG injection. These metabolic values were compared with myocardial flow distribution (%Flow), LVEF, LV volumes, and LV wall thickening (WT) determined by gated myocardial single-photon emission computed tomography using QGS software in eight myocardial segments.
Results
MGU correlated positively with LV volumes and negatively with LVEF. K1 was significantly higher in the segments of the patients than in those of the normal volunteers (0.082±0.055 vs 0.041±0.017 ml min−1 g−1, p<0.05), although there was no difference in MGU between the groups. On the other hand, SUV, k2, and k3 did not differ significantly between the groups. Among the patients, the K1 values were significantly higher in the areas with impaired WT (%WT<17%) (0.109±0.063 vs 0.069±0.062 ml min−1 g−1, p<0.05) and in the areas with flow reduction (%Flow<71%) (0.112±0.076 vs 0.071±0.046 ml min−1 g−1, p<0.05).
Conclusion
These results indicate that glucose utilization was preserved in the patients with LV dysfunction, mainly due to an increase in glucose transport, particularly in the regions with severely impaired LV function. Thus, the quantitative assessment of myocardial glucose utilization by FDG dynamic PET may provide useful information for assessing the regional myocardial metabolic status in patients with LV dysfunction.

Similar content being viewed by others
References
Marshall RC, Tillisch JH, Phelps ME, Huang SC, Carson R, Henze E, et al. Identification and differentiation of resting myocardial ischemia and infarction in man with positron computed tomography, 18F-labeled fluorodeoxyglucose and N-13 ammonia. Circulation 1983;67:766–78.
Tillisch J, Brunken R, Marshall R, Schwaiger M, Mandelkern M, Phelps M, et al. Reversibility of cardiac wall-motion abnormalities predicted by positron tomography. N Engl J Med 1986;314:884–8.
Schwaiger M, Brunken R, Grover-McKay M, Krivokapich J, Child J, Tillisch JH, et al. Regional myocardial metabolism in patients with acute myocardial infarction assessed by positron emission tomography. J Am Coll Cardiol 1986;8:800–8.
Brunken R, Schwaiger M, Grover-McKay M, Phelps ME, Tillisch J, Schelbert HR. Positron emission tomography detects tissue metabolic activity in myocardial segments with persistent thallium perfusion defects. J Am Coll Cardiol 1987;10:557–67.
Tamaki N, Yonekura Y, Yamashita K, Senda M, Saji H, Hashimoto T, et al. Relation of left ventricular perfusion and wall motion with metabolic activity in persistent defects on thallium-201 tomography in healed myocardial infarction. Am J Cardiol 1988;62:202–8.
Camici P, Ferrannini E, Opie LH. Myocardial metabolism in ischemic heart disease: basic principles and application to imaging by positron emission tomography. Prog Cardiovasc Dis 1989;32:217–38.
Bonow RO, Dilsizian V, Cuocolo A, Bacharach SL. Identification of viable myocardium in patients with chronic coronary artery disease and left ventricular dysfunction. Comparison of thallium scintigraphy with reinjection and PET imaging with 18F-fluorodeoxyglucose. Circulation 1991;83:26–37.
Tamaki N, Kawamoto M, Tadamura E, Magata Y, Yonekura Y, Nohara R, et al. Prediction of reversible ischemia after revascularization. Perfusion and metabolic studies with positron emission tomography. Circulation 1995;91:1697–1705.
Depre C, Vanoverschelde JL, Gerber B, Borgers M, Melin JA, Dion R. Correlation of functional recovery with myocardial blood flow, glucose uptake, and morphologic features in patients with chronic left ventricular ischemic dysfunction undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 1997;113:371–78.
Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation 1999;99:578–88.
McFalls EO, Baldwin D, Palmer B, Marx D, Jaimes D, Ward HB. Regional glucose uptake within hypoperfused swine myocardium as measured by positron emission tomography. Am J Physiol 1997;272:H343–9.
Young LH, Russell RR 3rd, Yin R, Caplan MJ, Ren J, Bergeron R, et al. Regulation of myocardial glucose uptake and transport during ischemia and energetic stress. Am J Cardiol 1999;83:25H–30H.
Young LH, Coven DL, Russell RR 3rd. Cellular and molecular regulation of cardiac glucose transport. J Nucl Cardiol 2000;7:267–76.
McFalls EO, Murad B, Liow JS, Gannon MC, Haspel HC, Lange A, et al. Glucose uptake and glycogen levels are increased in pig heart after repetitive ischemia. Am J Physiol Heart Circ Physiol 2002;282:H205–11.
Brosius FC 3rd, Liu Y, Nguyen N, Sun D, Bartlett J, Schwaiger M. Persistent myocardial ischemia increases GLUT1 glucose transporter expression in both ischemic and non-ischemic heart regions. J Mol Cell Cardiol 1997;29:1675–85.
Zorzano A, Sevilla L, Camps M, Becker C, Meyer J, Kammermeier H, et al. Regulation of glucose transport, and glucose transporter expression and trafficking in the heart: studies in cardiac myocytes. Am J Cardiol 1997;80:65A–76A.
Paternostro G, Pagano D, Gnecchi-Ruscone T, Bonser RS, Camici PG. Insulin resistance in patients with cardiac hypertrophy. Cardiovasc Res 1999;42:246–53.
Southworth R, Dearling JL, Medina RA, Flynn AA, Pedley RB, Garlick PB. Dissociation of glucose tracer uptake and glucose transporter distribution in the regionally ischaemic isolated rat heart: application of a new autoradiographic technique. Eur J Nucl Med Mol Imaging 2002;29:1334–41.
Taegtmeyer H. Switching metabolic genes to build a better heart. Circulation 2002;106:2043–5.
McFalls EO, Murad B, Haspel HC, Marx D, Sikora J, Ward HB. Myocardial glucose uptake after dobutamine stress in chronic hibernating swine myocardium. J Nucl Cardiol 2003;10:385–94.
Russell RR, 3rd, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 1999;277:H643–9.
Ratib O, Phelps ME, Huang SC, Henze E, Selin CE, Schelbert HR. Positron tomography with deoxyglucose for estimating local myocardial glucose metabolism. J Nucl Med 1982;23:577–86.
Gambhir SS, Schwaiger M, Huang SC, Krivokapich J, Schelbert HR, Nienaber CA, et al. Simple noninvasive quantification method for measuring myocardial glucose utilization in humans employing positron emission tomography and fluorine-18 deoxyglucose. J Nucl Med 1989;30:359–66.
Choi Y, Hawkins RA, Huang SC, Gambhir SS, Brunken RC, Phelps ME, et al. Parametric images of myocardial metabolic rate of glucose generated from dynamic cardiac PET and 2-[18F]fluoro-2-deoxy-d-glucose studies. J Nucl Med 1991;32:733–8.
Hicks RJ, Herman WH, Kalff V, Molina E, Wolfe ER, Hutchins G, et al. Quantitative evaluation of regional substrate metabolism in the human heart by positron emission tomography. J Am Coll Cardiol 1991;18:101–11.
Marinho NV, Keogh BE, Costa DC, Lammerstma AA, Ell PJ, Camici PG. Pathophysiology of chronic left ventricular dysfunction. New insights from the measurement of absolute myocardial blood flow and glucose utilization. Circulation 1996;93:737–44.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab 1985;5:584–90.
Germano G, Erel J, Lewin H, Kavanagh PB, Berman DS. Automatic quantitation of regional myocardial wall motion and thickening from gated technetium-99 m sestamibi myocardial perfusion single-photon emission computed tomography. J Am Coll Cardiol 1997;30:1360–7.
Sun D, Nguyen N, DeGrado TR, Schwaiger M, Brosius FC 3rd. Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation 1994;89:793–8.
Young LH, Renfu Y, Russell R, Hu X, Caplan M, Ren J, et al. Low-flow ischemia leads to translocation of canine heart GLUT-4 and GLUT-1 glucose transporters to the sarcolemma in vivo. Circulation 1997;95:415–22.
Brosius FC 3rd, Nguyen N, Egert S, Lin Z, Deeb GM, Haas F, et al. Increased sarcolemmal glucose transporter abundance in myocardial ischemia. Am J Cardiol 1997;80:77A–84A.
Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest 1999;104:1703–14.
Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation 2002;106:2125–31.
Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation 2002;106:407–11.
Tamaki N, Yonekura Y, Kawamoto M, Magata Y, Sasayama S, Takahashi N, et al. Simple quantification of regional myocardial uptake of fluorine-18-deoxyglucose in the fasting condition. J Nucl Med 1991;32:2152–7.
Yamaji S, Ishii K, Sasaki M, Mori T, Kitagaki H, Sakamoto S, et al. Evaluation of standardized uptake value to assess cerebral glucose metabolism. Clin Nucl Med 2000;25:11–6.
Knuuti MJ, Nuutila P, Ruotsalainen U, Saraste M, Harkonen R, Ahonen A, et al. Euglycemic hyperinsulinemic clamp and oral glucose load in stimulating myocardial glucose utilization during positron emission tomography. J Nucl Med 1992;33:1255–62.
Egert S, Nguyen N, Schwaiger M. Myocardial glucose transporter GLUT1: translocation induced by insulin and ischemia. J Mol Cell Cardiol 1999;31:1337–44.
Hashimoto K, Nishimura T, Imahashi KI, Yamaguchi H, Hori M, Kusuoka H. Lumped constant for deoxyglucose is decreased when myocardial glucose uptake is enhanced. Am J Physiol 1999;276:H129–33.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morita, K., Katoh, C., Yoshinaga, K. et al. Quantitative analysis of myocardial glucose utilization in patients with left ventricular dysfunction by means of 18F-FDG dynamic positron tomography and three-compartment analysis. Eur J Nucl Med Mol Imaging 32, 806–812 (2005). https://doi.org/10.1007/s00259-004-1743-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1743-2