Abstract
Purpose
Previous studies using dopamine transporter single-photon emission computed tomography (SPECT) to try and distinguish between patients with idiopathic Parkinson’s disease (IPD) and patients with atypical parkinsonian syndromes (APS) have mainly focussed on patients with an already established clinical diagnosis of several years’ duration. Differences in the pattern of striatal involvement between IPD and APS have been found in only few studies. We hypothesized that distinguishing SPECT features might be most pronounced at an early disease stage, and the purpose of the present study was to investigate this hypothesis.
Methods
The study included 72 patients with an initial clinical diagnosis of IPD, supported by decreased striatal [123I]β-CIT binding on baseline SPECT. In ten patients, the diagnosis was changed to APS over a mean follow-up period of 62 months. We retrospectively compared the patterns of striatal involvement on the baseline SPECT scans between the group of patients (re)diagnosed with APS and the remaining 62 patients in whom a diagnosis of IPD was maintained.
Results
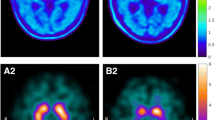
In the group of patients with APS, baseline [123I]β-CIT binding in both caudate nuclei was lower than in the group of patients with IPD. In addition, putamen to caudate binding ratios were higher in the group of APS patients. In spite of these differences, individual binding values showed considerable overlap between the groups.
Conclusion
[123I]β-CIT SPECT scanning in early-stage, untreated parkinsonian patients revealed a relative sparing of the caudate nucleus in patients with IPD as compared to patients later (re)diagnosed with APS. Nevertheless, the pattern of striatal involvement appears to have little predictive value for a later re-diagnosis of APS in individual cases.




Similar content being viewed by others
References
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–4.
Rajput AH, Rozdilsky B, Rajput A. Accuracy of clinical diagnosis in parkinsonism—a prospective study. Can J Neurol Sci 1991;18:275–8.
Hughes AJ, Daniel SE, Lees AJ. Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 2001;57:1497–9.
Hughes AJ, Daniel SE, Ben Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002;125:861–70.
Tissingh G, Bergmans P, Booij J, Winogrodzka A, van Royen EA, Stoof JC, et al. Drug-naive patients with Parkinson’s disease in Hoehn and Yahr stages I and II show a bilateral decrease in striatal dopamine transporters as revealed by [123I]β-CIT SPECT. J Neurol 1998;245:14–20.
Marek KL, Seibyl JP, Zoghbi SS, Zea-Ponce Y, Baldwin RM, Fussell B, et al. [123I]β-CIT/SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology 1996;46:231–7.
Innis RB, Seibyl JP, Scanley BE, Laruelle M, Abi-Dargham A, Wallace E, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson’s disease. Proc Natl Acad Sci USA 1993;90:11965–9.
Pirker W, Djamshidian S, Asenbaum S, Gerschlager W, Tribl G, Hoffmann M, et al. Progression of dopaminergic degeneration in Parkinson’s disease and atypical parkinsonism: a longitudinal β-CIT SPECT study. Mov Disord 2002;17:45–53.
Asenbaum S, Brücke T, Pirker W, Podreka I, Angelberger P, Wenger S, et al. Imaging of dopamine transporters with iodine-123-β-CIT and SPECT in Parkinson’s disease. J Nucl Med 1997;38:1–6.
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, et al. Decreased single-photon emission computed tomographic [123I]β-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 1995;38:589–98.
Varrone A, Marek KL, Jennings D, Innis RB, Seibyl JP. [123I]β-CIT SPECT imaging demonstrates reduced density of striatal dopamine transporters in Parkinson’s disease and multiple system atrophy. Mov Disord 2001;16:1023–32.
Antonini A, Benti R, De Notaris R, Tesei S, Zecchinelli A, Sacilotto G, et al. 123I-Ioflupane/SPECT binding to striatal dopamine transporter (DAT) uptake in patients with Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Neurol Sci 2003;24:149–50.
Messa C, Volonte MA, Fazio F, Zito F, Carpinelli A, d’Amico A, et al. Differential distribution of striatal [123I]β-CIT in Parkinson’s disease and progressive supranuclear palsy, evaluated with single-photon emission tomography. Eur J Nucl Med 1998;25:1270–6.
Brücke T, Asenbaum S, Pirker W, Djamshidian S, Wenger S, Wober C, et al. Measurement of the dopaminergic degeneration in Parkinson’s disease with [123I]β-CIT and SPECT. Correlation with clinical findings and comparison with multiple system atrophy and progressive supranuclear palsy. J Neural Transm Suppl 1997;50:9–24.
Pirker W, Asenbaum S, Bencsits G, Prayer D, Gerschlager W, Deecke L, et al. [123I]β-CIT SPECT in multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Mov Disord 2000;15:1158–67.
Kim YJ, Ichise M, Ballinger JR, Vines D, Erami SS, Tatschida T, et al. Combination of dopamine transporter and D2 receptor SPECT in the diagnostic evaluation of PD, MSA, and PSP. Mov Disord 2002;17:303–12.
Nurmi E, Bergman J, Eskola O, Solin O, Vahlberg T, Sonninen P, et al. Progression of dopaminergic hypofunction in striatal subregions in Parkinson’s disease using [18F]CFT PET. Synapse 2003;48:109–15.
Innis RB, Marek KL, Sheff K, Zoghbi S, Castronuovo J, Feigin A, et al. Effect of treatment with L-dopa/carbidopa or L-selegiline on striatal dopamine transporter SPECT imaging with [123I]β-CIT. Mov Disord 1999;14:436–42.
Ahlskog JE, Uitti RJ, O’Connor MK, Maraganore DM, Matsumoto JY, Stark KF, et al. The effect of dopamine agonist therapy on dopamine transporter imaging in Parkinson’s disease. Mov Disord 1999;14:940–6.
Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745–52.
Fahn S, Elthon RL, and members of the UPDRS Development Committee. The Unified Parkinson’s Disease Rating Scale. Recent developments in Parkinson’s disease, Vol 2. Florham Park, NY: Macmillan Healthcare Information, 1987, pp 153–163.
Booij J, Tissingh G, Boer GJ, Speelman JD, Stoof JC, Janssen AG, et al. [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 1997;62:133–40.
Gilman S, Low PA, Quinn N, Albanese A, Ben Shlomo Y, Fowler CJ, et al. Consensus statement on the diagnosis of multiple system atrophy. J Auton Nerv Syst 1998;74:189–92.
Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 1996;47:1–9.
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113–24.
Tissingh G, Bergmans P, Booij J, Winogrodzka A, Stoof JC, Wolters EC, et al. [123I]β-CIT single-photon emission tomography in Parkinson’s disease reveals a smaller decline in dopamine transporters with age than in controls. Eur J Nucl Med 1997;24:1171–4.
Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–42.
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 1973;20:415–55.
Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med 1988;318:876–80.
Kish SJ, Chang LJ, Mirchandani L, Shannak K, Hornykiewicz O. Progressive supranuclear palsy: relationship between extrapyramidal disturbances, dementia, and brain neurotransmitter markers. Ann Neurol 1985;18:530–6.
Ruberg M, Javoy-Agid F, Hirsch E, Scatton B, LHeureux R, Hauw JJ, et al. Dopaminergic and cholinergic lesions in progressive supranuclear palsy. Ann Neurol 1985;18:523–9.
Goto S, Hirano A, Matsumoto S. Subdivisional involvement of nigrostriatal loop in idiopathic Parkinson’s disease and striatonigral degeneration. Ann Neurol 1989;26:766–70.
Tissingh G, Booij J, Bergmans P, Winogrodzka A, Janssen AG, van Royen EA, et al. Iodine-123-N-Ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J Nucl Med 1998;39:1143–8.
Ilgin N, Zubieta J, Reich SG, Dannals RF, Ravert HT, Frost JJ. PET imaging of the dopamine transporter in progressive supranuclear palsy and Parkinson’s disease. Neurology 1999;52:1221–6.
Schwarz J, Tatsch K, Gasser T, Arnold G, Pogarell O, Kunig G, et al. 123I-IBZM binding compared with long-term clinical follow up in patients with de novo parkinsonism. Mov Disord 1998;13:16–9.
Acknowledgements
The financial support of the Dutch Parkinson Foundation (Parkinson Patiënten Vereniging) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stoffers, D., Booij, J., Bosscher, L. et al. Early-stage [123I]β-CIT SPECT and long-term clinical follow-up in patients with an initial diagnosis of Parkinson’s disease. Eur J Nucl Med Mol Imaging 32, 689–695 (2005). https://doi.org/10.1007/s00259-004-1733-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1733-4