Abstract
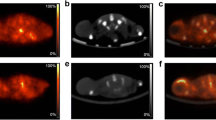
Carbon-11 choline has recently been introduced as a potential tracer for tumour imaging with positron emission tomography (PET). We evaluated the kinetics of the uptake of [11C]choline in prostate cancer and benign prostatic hyperplasia. We also evaluated the association between the uptake of [11C]choline and the histological grade of malignancy, Gleason score, volume of the prostate and prostate-specific antigen (PSA). Fourteen patients with histologically confirmed prostate cancer and five patients with benign prostatic hyperplasia were studied with [11C]choline PET. A mean dose of 430±31 MBq of [11C]choline was injected intravenously and a dynamic emission acquisition of prostate was performed for 30 min. The uptake of [11C]choline was measured as a standardised uptake value (SUV) and as a kinetic influx constant (K i) obtained from graphical analysis. Both cancerous and hyperplastic prostate were well visualised with [11C]choline against low or moderate tracer accumulation in the bladder and rectal wall. The measured radioactivity in urine was invariably low. In the graphical analysis, linear plots were achieved. The mean K i of the untreated tumour was 0.205±0.089 min−1 (range 0.128–0.351; n=7) and the mean SUV was 5.6±3.2 (range 1.9–15.5; n=15). K i values and SUVs correlated closely (r=0.964, P=0.0005), whereas no correlation could be demonstrated between the tumour uptake of [11C]choline and the histological grade, Gleason score, volume of the prostate or PSA . The mean SUV and the mean K i of benign hyperplastic prostate were 3.5±1.0 (range 2.0–4.5; n=4) and 0.119±0.076 min−1 (range 0.065–0.173; n=2). In conclusion, a high uptake of [11C]choline characterises not only carcinomatous but also hyperplastic prostatic tissue. Dynamic imaging of the uptake of [11C]choline in the prostate shows a good applicability of the graphical analysis model with an irreversible compartment. A close correlation between the K i values and semiquantitative SUVs of tumours supports the use of the simpler SUV in the clinical setting.


Similar content being viewed by others
References
Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics 1996. CA Cancer J Clin 1996; 65:5–27.
Yu KK, Hricak H. Imaging prostate cancer. Radiol Clin North Am 2000; 38:59–85.
May F, Treumann T, Dettmar P, Hartung R, Breul J. Limited value of endorectal magnetic resonance imaging and transrectal ultrasonography in the staging of clinically localized prostate cancer. BJU Int 2001; 87:66–69.
Hricak H, Dooms GC, Jeffrey RB, et al. Prostatic carcinoma: staging by clinical assessment, CT, and MRI imaging. Radiology 1987; 162:331–336.
Smith PH, Bono A, Calais da Silva F, et al. Some limitations of the radioisotope bone scan in patients with metastatic prostatic cancer. Cancer 1990; 66:1009–1016.
Tiguert R, Gheiler EL, Tefilli MV, et al. Lymph node size does not correlate with the presence of prostate cancer metastasis. Urology 1999; 53:367–371.
Carroll P, Coley C, McLeod D, et al. Prostate-specific antigen best practice policy. Part I. Early detection and diagnosis of prostate cancer. Urology 2001; 57:217–224.
Carroll P, Coley C, McLeod D, et al. Prostate-specific antigen best practice policy. Part II. Prostate cancer staging and post-treatment follow-up. Urology 2001; 57:225–229.
Gambhir SS, Czernin J, Schwimmer J, Silverman DHS, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med 2001; 42:1S–93S.
Effert PJ, Bares R, Handt S, Wolff JM, Bull U, Jakse G. Metabolic imaging of untreated prostate cancer by positron emission tomography with18fluorine-labeled deoxyglucose. J Urol 1996; 155:994–998.
Hofer C, Laubenbacher C, Block T, Breul J, Hartung R, Schwaiger M. Fluorine-18-fluorodeoxyglucose positron emission tomography is useless for the detection of local recurrence after radical prostatectomy. Eur Urol 1999; 36:31–35.
Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 2001; 57:108–111.
Hoh CK, Seltzer MA, Franklin J, deKernion JB, Phelps ME, Belldegrun A. Positron emission tomography in urological oncology. J Urol 1998; 159:347–356.
Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon-11-choline. J Nucl Med 1998; 39:990–995.
Kotzerke J, Prang J, Neumaier B, et al. Experience with carbon-11-choline positron emission tomography in prostate cancer. Eur J Nucl Med 2000; 27:1415–1419.
De Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensink HJA. Visualization of prostate cancer with11C-choline positron emission tomography. Eur Urol 2002; 42:18–23.
De Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensik HJ. Preoperative staging of pelvic lymph nodes in prostate cancer by11C-choline PET. J Nucl Med 2003; 44:331–335.
De Jong IJ, Pruim J, Elsinga PH, Vaalburg W, Mensik HJA.11C-Choline positron emission tomography for the evaluation after treatment of localized prostate cancer. Eur Urol 2003; 44:32–39.
Picchio M, Messa C, Landoni C, Gianolli L, Sironi S, Brioschi M, Matarrese M, Matei DV, De Cobelli F, Del Maschio A, Rocco F, Rigatti P, Fazio F. Value of [11C]choline-positron emission tomography for re-staging prostate cancer: a comparison with [18F]fluorodeoxyglucose-positron emission tomography. J Urol 2003; 169:1337–1340.
Zeisel SH. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr 1981; 1:95–121.
Negendank W. Studies of human tumors by MRS: a review. NMR Biomed 1992; 5:303–324.
Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ. Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24–0.7 cm3) spatial resolution. Radiology 1996; 198:795–805.
Bhakoo KK, Williams SR, Florian CL, Land H, Noble MD. Immortalization and transformation are associated with specific alterations in choline metabolism. Cancer Res 1996; 56:4630–4635.
Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res 2001; 61:3599–3603.
Hernandez-Alcoceba R, Saniger L, Campos J, et al. Choline kinase inhibitors as a novel approach for antiproliferative drug design. Oncogene 1997; 15:2289–2301.
Molina AR, Penalva V, Lucas L, Lacal JC. Regulation of choline kinase activity by RAS proteins involves Ra1-GDS and PI3 K. Oncogene 2002; 21:937–946.
Sobin LH, Wittekind CH, eds. TNM classification of malignant tumours, 5th edn. New York: Wiley; 1997:170–173.
Mostofi FK, Sesterhenn I, Sobin LH. International histological classification of tumours, No. 22. Histological typing of prostate tumours. Geneva: World Health Organization; 1980:7–26.
Gleason DF, for the Veterans Administration Cooperative Urological Group. Histologic grading and staging of prostatic carcinoma. In: Tannenbaum M, ed. Urologic pathology: The prostate. Philadelphia: Lea and Febiger; 1977:171–198.
Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol 1991; 145:984–987.
Roivainen A, Forsback S, Grönroos T, et al. Blood metabolism of [methyl-11C]choline; implications for in vivo imaging with positron emission tomography. Eur J Nucl Med 2000; 27:25–32.
Woodard HQ, Bigler RE, Freed B. Expression of tissue isotope distribution. J Nucl Med 1975; 16:958–959.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Metab 1985; 5:584–590.
Oyama N, Akino H, Kanamaru H, et al.11C-acetate PET imaging of prostate cancer. J Nucl Med 2002; 43:181–186.
Kato T, Tsukamoto E, Kuge Y et al. Accumulation of [11C]acetate in normal prostate and benign prostatic hyperplasia: comparison with prostate cancer. Eur J Nucl Med Mol Imaging 2002; 29:1492–1495.
DeGrado TR, Coleman RE, Wang S, et al. Synthesis and evaluation of18F-labeled choline as an oncologic tracer for positron emission tomography: initial findings in prostate cancer. Cancer Res 2000; 61:110–117.
Hara T, Kosaka N, Kishi H. Development of18F-fluoroethylcholine for cancer imaging with PET: synthesis, biochemistry, and prostate cancer imaging. J Nucl Med 2002; 43:187–199.
Ward JF, Morris JC, Hamblen S, et al. Prostate cancer imaging in murine model using carbon-11 labeled choline and acetate [abstract]. Mol Imaging Biol 2002; 4S:S35.
Kotzerke J, Volkmer BG, Glatting G, van den Hoff J, Gschwend JE, Messer P, Reske SN, Neumaier B. Intraindividual comparison of [11C]acetate and [11C]choline PET for detection of metastases of prostate cancer. Nuklearmedizin 2003; 42:25–30.
Acknowledgements
We thank the personnel of the Turku PET Centre, Departments of Nuclear Medicine, Oncology and Radiotherapy, and Urology, for their assistance and pleasant co-operation. We also thank Lauri Sillanmäki and Heikki Hiekkanen, from the Department of Biostatistics, for their help. This study was financially supported by the Turku University Foundation and The Finnish Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sutinen, E., Nurmi, M., Roivainen, A. et al. Kinetics of [11C]choline uptake in prostate cancer: a PET stydy. Eur J Nucl Med Mol Imaging 31, 317–324 (2004). https://doi.org/10.1007/s00259-003-1377-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1377-9