Abstract
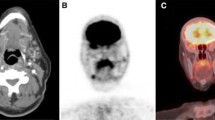
Several studies have reported on the expression of somatostatin receptors in patients with differentiated thyroid cancer (DTC). The aim of this study was to evaluate the imaging abilities of a recently developed technetium-99m labelled somatostatin analogue, 99mTc-EDDA/HYNIC-TOC (99mTc-TOC), in terms of precise localisation of disease. The study population comprised 54 patients (24 men, 30 women; age range 22–90 years) with histologically confirmed DTC who presented with recurrent or persistent disease as indicated by elevated Tg levels after initial treatment. All patients were negative on the iodine-131 post-therapy whole-body scans. Fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET) was performed in a subgroup of 36 patients. The study population consisted of two groups: Group A (n=22) comprised patients with disease recurrence as shown by elevated Tg levels but without detectable pathology. In group B (n=32), pre-existing lesions were known. Among the 54 cases, SSTR scintigraphy was true positive in 33 (61.1%), true negative in 4 (7.4%) and false negative in 17 (31.5%) cases, which resulted in a sensitivity of 66%. A total of 138 tumour foci were localised in 33 patients. The fraction of true positive 99mTc-TOC findings was positively correlated (P<0.01) with elevated Tg levels (higher than 30 ng/ml). Despite two false positive findings, analysis on a lesion basis demonstrated better diagnostic efficacy with 18F-FDG PET (P<0.001); however, it also revealed substantial agreement between the imaging techniques [Cohen’s kappa of 0.62 (0.47–0.78)]. In conclusion, scintigraphy with 99mTc-TOC might be a promising tool for treatment planning; it is easy to perform and showed sufficient accuracy for localisation diagnostics in thyroid cancer patients with recurrent or metastatic disease.






Similar content being viewed by others
References
Pacini F, DeGroot LJ. Thyroid neoplasia. In: DeGroot LJ, Jameson JL, eds. Endocrinology, 4th edn. Philadelphia: Saunders; 2001:1541–1566.
Ronga G, Fiorentino A, Paserio E, et al. Can iodine-131 whole-body scan be replaced by thyroglobulin measurement in the post surgical follow-up of differentiated thyroid carcinoma. J Nucl Med 1990; 31:1766–1771.
Ozata M, Suzuki S, Miyamoto T, Tsuan Liu RT, Fierro-Renoy F, Degroot LJ. Serum thyroglobulin in the follow-up of patients with treated differentiated thyroid cancer. J Clin Endocrinol Metab 1994; 79:98–105.
Lubin E, Mechli-Frish S, Zatz S, et al. Serum thyroglobulin and iodine-131 whole-body scan in the diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J Nucl Med 1994; 35:257–262.
Schlumberger MJ. Diagnostic follow-up of well-differentiated thyroid carcinoma: historical perspective and current status. J Endocrinol Invest 1999; 22 (11 Suppl):3–7.
Feine U, Lietzenmayer R, Hanke JP, Held J, Woehrle H, Mueller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med 1996; 37:1468–1472.
Schlueter B, Bohuslavizki KH, Beyer W, Plotkin M, Buchert R, Clausen M. Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative131I scan. J Nucl Med 2001; 42:71–76.
Helal BO, Merlet P, Toubert ME, et al. Clinical impact of18F-FDG PET in thyroid carcinoma patients with elevated thyroglobulin levels and negative 131I scanning results after therapy. J Nucl Med 2001; 42:1464–1469.
Briele B, Hotze A, Kroopp J, et al. A comparison of201Tl and 99mTc-MIBI in the follow-up of differentiated thyroid carcinomas. Nuklearmedizin 1991; 30:115–124.
Lind P, Gallowitsch HJ, Langsteger W, Kresnik E, Mikosch P, Gomez I. Technetium-99m-tetrofosmin whole-body scintigraphy in the follow-up of differentiated thyroid carcinoma. J Nucl Med 1997; 38:348–354.
Miyamoto S, Kasagi K, Misaki T, Alam MS, Konishi J. Evaluation of technetium-99m-MIBI scintigraphy in metastatic differentiated thyroid carcinoma. J Nucl Med 1997; 38:352–356.
Rubello D, Mazzarotte R, Casara D. The role of technetium-99m methoxyisobutylisonitrile scintigraphy in the planning of therapy and follow-up of patients with differentiated thyroid carcinoma after surgery. Eur J Nucl Med 2000; 27:431–440.
Ng DCE, Sundram FX, Sin AE.99mTc-sestamibi and 131I whole-body scintigraphy and initial serum thyroglobulin in the management of differentiated thyroid carcinoma. J Nucl Med 2000; 41:631–635.
Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111-In-DTPA-d-Phe] and [123-I-Tyr]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993; 20:716–731.
Krenning EP, Kwekkeboom DJ, de Jong M, et al. Essentials of peptide receptor scintigraphy with emphasis on somatostatin analog octreotide. Semin Oncol 1994; 21 (Suppl 13):6–14.
Lamberts SWJ, Reubi JC, Krenning EP. Somatostatin and the concept of peptide receptor scintigraphy in oncology. Semin Oncol 1994; 21 (Suppl) 13:1–5.
Kwekkeboom D, Krenning EP, de Jong M. Peptide receptor imaging and therapy. J Nucl Med 2000; 41:1704–1713.
Balon HR, Goldsmith SJ, Siegel BA, et al. Procedure guidelines for somatostatin receptor scintigraphy with111In-pentetreotide. J Nucl Med 2001; 42:1134–1138.
Tenenbaum F, Lumbroso J, Schlumberger M, Caillou B, Fragu P, Parmentier C. Radiolabeled somatostatin analog scintigraphy in differentiated thyroid carcinoma. J Nucl Med 1995; 36:807–810.
Baudin E, Schlumberger M, Lumbroso J, Travagli JP, Caillou B, Parmentier C. Octreotide scintigraphy in patients with differentiated thyroid carcinoma: contribution for patients with negative radioidine scan. J Clin Endocrinol Metab 1996; 81:2541–2544.
Ahlman H, Tisell LE, Wangberg B, et al. The relevance of somatostatin receptors in thyroid neoplasia. Yale J Biol Med 1997; 70:523–533.
Gulec SA, Serafini AN, Sridhar KS, et al. Somatostatin receptor expression in Hürthle cell cancer of the thyroid. J Nucl Med 1998; 39:243–245.
Garin E, Devillers A, Le Cloirec J, et al. Use of indium-111 pentetreotide somatostatin receptor scintigraphy to detect recurrent thyroid carcinoma in patients without detectable iodine uptake. Eur J Nucl Med 1998; 25:687–694.
Kolby L, Wangberg B, Ahlman H, et al. Somatostatin receptor subtypes, octreotide scintigraphy, and clinical response to octreotide treatment in patients with neuroendocrine tumors. World J Surg 1998; 22:679–683.
Valli N, Catargi B, Ronci N, et al. Evaluation of indium-111 pentetreotide somatostatin receptor scintigraphy to detect recurrent thyroid carcinoma in patients with negative radioiodine scintigraphy. Thyroid 1999; 9:583–589.
Haslinghuis LM, Krenning EP, De Herder WW, Reijs AE, Kwekkeboom DJ. Somatostatin receptor scintigraphy in the follow-up of patients with differentiated thyroid cancer. J Endocrinol Invest 2001; 24:415–422.
Goerges R, Kahaly G, Mueller-Brand J, Maecke H, Roser HW, Bockisch A. Radionuclide-labeled somatostatin analogues for diagnostic and therapeutic purposes in nonmedullary thyroid cancer. Thyroid 2001; 11:647–659.
Forssell-Aronsson EB, Nilsson O, Benjegard A, et al.111In-DTPA-d-Phe1-octreotide binding and somatostatin receptor subtypes in thyroid tumors. J Nucl Med 2000; 41:636–642.
Decristoforo C, Melendez-Alafort L, Sosabowski JK, Mather SJ.99mTc-HYNIC-[Tyr3]-octreotide for imaging somatostatin-receptor-positive tumors: preclinical evaluation and comparison with 111In-octreotide. J Nucl Med 2000; 41:1114–1119.
Decristoforo C, Mather SJ, Cholewinski W, Donnemiller E, Riccabona G, Moncayo R.99mTc-EDDA/HYNIC-TOC: a new 99mTc-labelled radiopharmaceutical for imaging somatostatin receptor-positive tumours: first clinical results and intra-patient comparison with 111In-labelled octreotide derivates. Eur J Nucl Med 2000; 27:1318–1325.
Gabriel M, Decristoforo C, Donnemiller E, et al. An intrapatient comparison of99mTc-EDDA/HYNIC-TOC with 111In-DTPA-octreotide for diagnosis of somatostatin receptor expressing tumors. J Nucl Med 2003; 44:708–716.
Sobin LH, Wittekind C. International Union Against Cancer. TNM classification of malignant tumours, 5th edn. New York: Wiley-Liss, 1997.
American Joint Committee on Cancer. AJCC cancer staging manual, 5th edn. In: Part II: 8. Thyroid Gland. Philadelphia, New York: Lippincott-Raven; 1997:59–61.
Decristoforo C, Mather SJ. Preparation,99mTc-labelling and in vitro characterisation of HYNIC and N3S modified RC-160 and [Tyr3]-octreotide. Bioconjug Chem 1999; 10:431–438.
Le Rest C, Bomanji JB, Costa DC, Townsend CE, Visvikis D, Ell PJ. Functional imaging of malignant paragangliomas and carcinoid tumours. Eur J Nucl Med 2001; 28:478–482.
Petrich T, Boerner AR, Otto D, Hofmann M, Knapp WH. Influence of rhTSH on [18F]fluorodeoxyglucose uptake by differentiated thyroid carcinoma. Eur J Nucl Med Mol Imaging 2002; 29:641–647.
Wang W, Larson SM, Tuttle RM, et al. Resistance of [18F]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid 2001; 11:1169–1175.
Robbins RJ, Hill RH, Wang W, Macapinlac HH, Larson SM. Inhibition of metabolic activity in papillary thyroid carcinoma by a somatostatin analogue. Thyroid 2000; 10:177–183.
Arnold R, Simon B, Wied M. Treatment of neuroendocrine GEP tumours with somatostatin analogues: a review. Digestion 2000; 62 Suppl 1:84–91.
Waldherr C, Schumacher T, Pless M, et al. Radiopeptide transmitted internal irradiation of non-iodophil thyroid cancer and conventionally untreatable medullary thyroid cancer using [90Y]-DOTA-d-Phe1-Tyr3-octreotide: a pilot study. Nucl Med Commun 2001; 22:673–678.
Hsu CH, Liu FY, Yen RF, Kao CH. Tc-99m MIBI SPECT in detecting metastatic papillary thyroid carcinoma in patients with elevated human serum thyroglobulin levels but negative I-131 whole body scan. Endocr Res 2003; 29:9–15.
Rubello D, Piotto A, Pagetta C, Pelizzo MR, Casara D.99mTc-MIBI radio-guided surgery for recurrent thyroid carcinoma: technical feasibility and procedure, and preliminary clinical results. Eur J Nucl Med Mol Imaging 2002; 29:1201–1205.
Acknowledgements
We especially wish to thank Prof. G. Riccabona, Prof. Emeritus, University of Innsbruck, for his help and support in initiating this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gabriel, M., Froehlich, F., Decristoforo, C. et al. 99mTc-EDDA/HYNIC-TOC and 18F-FDG in thyroid cancer patients with negative 131I whole-body scans. Eur J Nucl Med Mol Imaging 31, 330–341 (2004). https://doi.org/10.1007/s00259-003-1376-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1376-x