Abstract
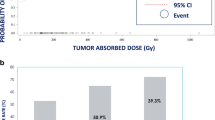
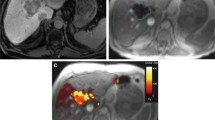
A multicentre study was sponsored by the International Atomic Energy Agency (Vienna) to assess the safety and efficacy of trans-arterial rhenium-188 HDD conjugated lipiodol (radioconjugate) in the treatment of patients with inoperable hepatocellular carcinoma (HCC). The radioconjugate was prepared by using an HDD (4-hexadecyl 1-2,9,9-tetramethyl-4,7-diaza-1,10-decanethiol) kit developed in Korea, and lipiodol. Over a period of 18 months, 70 patients received at least one treatment of radioconjugate. Some patients were re-treated if there was no evidence of disease progression. The level of radioconjugate administered was based on radiation-absorbed dose to critical normal organs, calculated following a “scout” dose of radioconjugate. The organs at greatest risk for radiation toxicity are the normal liver, the lung and the bone marrow. An Excel spreadsheet was used to determine maximum tolerated activity (MTA), defined as the amount of radioactivity calculated to deliver no more than 12 Gy to lungs, or 30 Gy to liver, or 1.5 Gy to bone marrow. These doses have been found to be safe in multiple trials using external beam therapy, but this has not been confirmed for systemically administered radiopharmaceuticals. Patients were followed for at least 12 weeks after therapy, until recovery from all toxicity. The clinical parameters evaluated included toxicity, response as determined by contrast-enhanced computed tomography, palliation of symptoms, overall survival, performance status (Karnofsky) and hepatic function (Child’s classification). Liver function tests, serum α-fetoprotein (AFP) levels and complete blood counts were done at each follow-up visit. In the majority of patients, the scout dose studies indicated the radiation absorbed dose to normal liver to be the limiting factor to the treatment dose, while in a few patients dose to lung was the limiting factor. Radiation dose to bone marrow was negligible and was thus not a factor for the MTA calculations. Side-effects were minimal and usually presented as loss of appetite, right hypochondrial discomfort and low-grade fever, even at high levels of administered radioactivity. The symptoms resolved with simple supportive therapy within 3 days of onset. Liver function tests at 24 and 72 h showed no significant changes and complete blood counts at 1 week, 4 weeks and 12 weeks showed no changes (no bone marrow suppression). Sixteen patients were treated in the dose escalation phase of the study, when the activities administered started at 1.8 GBq (50 mCi) and rose to 7.7 GBq (206 mCi). In the efficacy phase of the study a further 54 patients were treated. Both groups of patients are included in this paper. The treatment activity of 188Re-lipiodol administered transarterially ranged from 1.8 to 9.8 GBq (50–265 mCi), with a mean activity of 4.6 GBq (124 mCi). Survival at 3 months was 90%, and at 6 months, 60%; 19% survived for 1 year. Mean survival after treatment in the total treated group of 70 patients was 9.5 months, with a range of 1–18 months. The results of this multicentre study show that 188Re-lipiodol is a safe and cost-effective method to treat primary HCC via the transarterial route. In terms of efficacy, it is potentially a new therapeutic approach for further evaluation by treatment of larger numbers of patients.


Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94:153–156.
Akriviadis EA, Llvovel JM, Efremidis SC, et al. Hepatocellular carcinoma. Br J Surg 1998; 85:1319–1331.
Okuda K. Hepatocellular carcinoma: recent progress. Hepatology 1992; 15:948–963.
Ince N, Wands R. The increasing incidence of hepatocellular carcinoma. N Engl J Med 1999; 340:798–802.
Llovet JM, Bustamante J, Castells A, Velana R, et al. Natural history of unresected nonsurgical hepatocellular carcinoma for the design and evaluation of therapeutic trials. Hepatology 1999; 29:62–67.
Okuda K, Ohtsuki T. Obata H,et al. Natural history of hepatocellular carcinoma and progress in relation to treatment. Cancer 1985; 56:918.
Nagao T, Inoue S, Yochimi F, et al. Postoperative recurrence of hepatocellular carcinoma. Ann Surg 1990; 211:28–33.
Bruix J. Treatment of hepatocellular carcinoma. Hepatology 1997; 25:259.
Ohishi H, Uchida H, Yoshimura H, et al. Hepatocellular carcinoma detected by iodised oil. Use of anticancer agents. Radiology 1985; 154:25–29.
Knapp FF Jr, Beets AL, Guhlke S, Zamora PO, Bender H, Palmedo H, Biersack HJ. Development of the alumina-based tungsten-188/rhenium-188 generator and use of rhenium-188-labeled radiopharmaceuticals for cancer treatment. Anticancer Res 1997; 17:1783–1796.
Jeong JM, Kim YJ, Lee YS, et al. Lipiodol solution of a lipophilic agent, Re-188 TDD for the treatment of liver cancer. Nucl Med Biol 2001; 28:197–204.
Lee YS, Jeong JM, Kim YJ, et al. Synthesis of188Re-labelled long chain alkyl diaminedithiol for therapy of liver cancer. Nucl Med Commun 2002; 23:237–242.
Shinna S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer 1991; 68:1524–1530.
Livraghi T, Goldberg SN, Lazzaroni S, et al. Hepatocellular carcinoma radio-frequency ablation of medium and large lesions. Radiology 2000; 214:761–768.
Ho S, Lau WY, Leung TW, Johnson PJ. Internal radiation therapy for patients with primary or metastatic hepatic cancer: a review. Cancer 1998; 83:1894–1907.
Zhou XD, Yu YQ, Tang ZY. An 18 year study of cryosurgery in the treatment of primary liver cancer. Asian J Surg 1992; 15:43–47.
Groupe D’Etude et de Traitement du Carcinome Hepatocellulaire. A comparison of lipiodol chemoembolisation and conservative treatment for unresectable hepatocellular carcinoma. N Engl J Med 1995; 332:1256–1261.
Ishikura S, Ogino T, Puruse J, et al. Radiotherapy after transcatheter arterial chemoembolization for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Clin Oncol 2002; 25:189–193.
Yoo HS, Park CH, Suk JH, et al. Radioiodinated fatty acid ester in the management of hepatocellular carcinoma: preliminary findings. Cancer Chemother Pharmacol 1989; 23 (Suppl 1):S54–S58.
Lau WY, Leung TWT, Ho SKW, et al. Adjuvant intra-arterial iodine 131 labelled lipiodol for resectable hepatocellular carcinoma: a prospective randomised trial. Lancet 1999; 353:797–801.
Dancey JE, Shepard FA, Paul K, et al. Treatment of non-resectable hepatocellular carcinoma with intrahepatic90Y-microspheres. J Nucl Med 2000; 41:1673–1681.
Lau Y, Ho S, Leung TWT, et al. Selective internal radiation therapy for non-resectable hepatocellular carcinoma with intraarterial infusion of 90 yttrium microspheres. Int J Radiat Oncol Biol Phys 1998; 40:583–592.
Ho S, Lau WY, Leung TW, Johnson PJ. Internal radiation therapy for patients with primary or metastatic cancer: a review. Cancer 1998; 83:1894–1907.
Raoul JL, Guyader D, Bretagne JF, et al. Randomised controlled trial for hepatocellular carcinoma with portal vein thrombosis: intra-arterial iodine-131 iodised oil versus medical support. J Nucl Med 1994; 35:1782–1787.
Sundram FX, Yu SWK, Jeong JM, et al. 188 Rhenium-TDD-lipiodol treatment of inoperable primary hepatocelullar carcinoma—a case report. Ann Acad Med Singapore 2001; 30:542–545.
Keng GHW, Sundram FX, Yu S, et al. Preliminary experience in radionuclide therapy of HCC using hepatic intra-arterial radio-conjugates. Ann Acad Med Singapore 2002; 31:382–386.
Zanzonico P, Divgi C, Sundram F, et al. Maximum-tolerated activity treatment planning protocol for intrahepatic artery Re-188 lipiodol therapy of liver cancer. J Nucl Med 2002; 43:355P–356P.
Sundram F, Jeong J, Zanzonico P, et al. Transarterial rhenium-188 lipiodol in the treatment of inoperable hepatocellular carcinoma. An IAEA sponsored multi-centre phase 1 study. World J Nucl Med 2002; 5–11.
Chaudakshetrin P, Osorio M, Somanesan S, Sundram FX, Padhy AK, Divgi CR, Zanzonico PB. Rhenium-188-lipiodol therapy of liver cancer: optimization of conjugate-view imaging of Re188 for patient-specific dosimetry. J Nucl Med 2003; 44:1161P.
Acknowledgements
The authors are grateful to the International Atomic Energy Agency for initiating and coordinating this multicentre study, for provision of tungsten/rhenium generators from ORNL, for experts and research grants, and for assistance to M.Sc./Ph.D. students in the various partaking institutions.
We also wish to thank the directors of the hospitals who supported us in this research: Dr. Truong Van Viet, Director, Cho Ray Hospital, Ho Chi Minh City, Vietnam; Dr. Ts Mukhar, Director, First State Central University Hospital, Ulaanbaator, Mongolia; and Dr. R. Esguerra, Director, Fundacion Santa Fe Hospital, Bogota, Columbia.
The following are the members of the Radionuclide Therapy of Liver Cancer Multicentre Trial Group: Singapore (Singapore General Hospital): F.X. Sundram, Sidney Yu, S. Somanesan, J. Premaraj, Gilbert Keng; Mongolia (First State Central University Hospital, Ulaanbaatar): Peljee Onkhuudai, Lodonsharav Tsevelmaa, Chultem Lamjav, Sereegotov Erdenechimeg, Davagsumberel Last Gonchigsuren; Colombia (Fundacion Santa Fe, Bogota): Patricia Bernal, Margarita Osorio, R. Esguerra; Vietnam (Cho Ray Hospital, Ho Chi Minh City): Tran Chi Minh Chau, Huynh Duc Long, Nguyen Van Hoa, Nguyen Dinh Song Huy; IAEA experts: Maung Maung Saw (Singapore), Yan Rolland (Rennes, France), Russ Knapp Jr. (Oak Ridge, USA), Pat Zanzonico (New York, USA), Chaitan Divgi (New York, USA), Jae Min Jeong (Seoul, Korea), John Buscombe (London, UK), Harvey Turner (Fremantle, Australia), Andrew Martindale (Fremantle, Australia), J. Raoul (Rennes, France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sundram, F., Chau, T.C.M., Onkhuudai, P. et al. Preliminary results of transarterial rhenium-188 HDD lipiodol in the treatment of inoperable primary hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 31, 250–257 (2004). https://doi.org/10.1007/s00259-003-1363-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1363-2