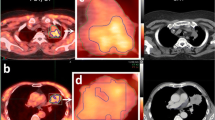
Abstract
Tumor hypoxia is recognized as an important determinant of response to therapy. In this study we investigated the feasibility of clinical imaging with copper-60 diacetyl-bis(N 4-methylthiosemicarbazone) (60Cu-ATSM) in patients with non-small-cell lung cancer (NSCLC) and also assessed whether pretreatment tumor uptake of 60Cu-ATSM predicts tumor responsiveness to therapy. Nineteen patients with biopsy-proved NSCLC were studied by positron emission tomography (PET) with 60Cu-ATSM before initiation of therapy. 60Cu-ATSM uptake was evaluated semiquantitatively by determining the tumor-to-muscle activity ratio (T/M). All patients also underwent PET with fluorine-18 fluorodeoxyglucose (FDG) prior to institution of therapy. The PET results were correlated with follow-up evaluation (2–46 months). It was demonstrated that PET imaging with 60Cu-ATSM in patients with NCSLC is feasible. The tumor of one patient had no discernible 60Cu-ATSM uptake, whereas the tumor uptake in the remaining patients was variable, as expected. Response was evaluated in 14 patients; the mean T/M for 60Cu-ATSM was significantly lower in responders (1.5±0.4) than in nonresponders (3.4±0.8) (P=0.002). However, the mean SUV for 60Cu-ATSM was not significantly different in responders (2.8±1.1) and nonresponders (3.5±1.0) (P=0.2). An arbitrarily selected T/M threshold of 3.0 discriminated those likely to respond to therapy: all eight responders had a T/M <3.0 and all six nonresponders had a T/M ≥3.0. Tumor SUV for FDG was not significantly different in responders and nonresponders (P=0.7) and did not correlate with 60Cu-ATSM uptake (r=0.04; P=0.9). 60Cu-ATSM-PET can be readily performed in patients with NSCLC and the tumor uptake of 60Cu-ATSM reveals clinically unique information about tumor oxygenation that is predictive of tumor response to therapy.



Similar content being viewed by others
References
Höckel M, Knoop C, Schlenger B et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 1993; 26:45–50.
Olive PL, Durand RE, Le Riche J, Olivotto IA, Jackson SM. Gel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancers. Cancer Res 1993; 53:733–746.
Olive PL, Vikse CM, Durand RE. Hypoxic fractions measured in murine tumors and normal tissues using the comet assay. Int J Radiat Oncol Biol Phys 1994; 29:487–491.
Evans SM, Jenkins WT, Joiner B, Lord EM, Koch CJ. 2-Nitroimidazole (EF5) binding predicts radiation resistance in individual 9L s.c. tumors. Cancer Res 1996; 56:405–411.
Evans SM, Joiner B, Jenkins WT, Laughlin KM, Lord EM, Koch CJ. Identification of hypoxia in cells and tissues of epigastric 9L rat glioma using EF5 [2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3-pentafluoropropyl) acetamide]. Br J Cancer 1995; 72:875–882.
Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 1996; 56:4509–4515.
Brizel DM, Scully SP, Harrelson JM et al. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res 1996; 56:5347–5350.
Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. J Radiother Oncol 1996; 41:31–39.
Jerabek PA, Patrick TB, Kilbourn MR, Dischino DD, Welch MJ. Synthesis and biodistribution of [F-18] labeled fluoromisonidazoles: potential in vivo markers of hypoxic tissue. Int J Radiat Appl Instrum 1986; Part A 37:599–605.
Grierson JR, Link JM, Mathis CA, Rasey JS, Krohn KA. A radiosynthesis of fluorine-18 fluoromisonidazole. J Nucl Med 1989; 30:343–350.
Koh W-J, Rasey JS, Evans ML. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992; 22:199–212.
Martin GV, Caldwell JH, Graham MM et al. Noninvasive detection of hypoxic myocardium using [F-18]fluoromisonidazole and positron emission tomography. J Nucl Med 1992; 33:2202–2208.
Rasey JS, Nelson NJ, Chin L, et al. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. Radiat Res 1990; 122:301–308.
Rischin D, Peters L, Hicks R et al. Phase I trial of concurrent tirapazamine, cisplatin, and radiotherapy in patients with advanced head and neck cancer. J Clin Oncol 2001; 19:535–542.
Fujibayashi Y, Taniuchi H, Yonekura Y, et al. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 1997; 38:1155–1160.
Fujibayashi Y, Cutler CS, Anderson CJ et al. Comparative studies of Cu-64-ATSM and C-11-acetate in an acute myocardial infarction model: ex vivo imaging of hypoxia in rats. Nucl Med Biol 1999; 26:117–121.
Lewis JS, McCarthy DW, McCarthy TJ, et al. The evaluation of64Cu-diacetyl-bis(N 4-methylthiosemicarbazone)(64Cu-ATSM) in vitro and in vivo in a hypoxic tumor model. J Nucl Med 1999; 40:177–183.
Lewis JS, Sharp TL, Laforest R, et al. Tumor uptake of copper-diacetyl-bis(N 4-methythiosemicarbazone): effect of changes in tissue oxygenation. J Nucl Med 2001; 42:655–661.
Alshanqueeti A, Kaplan C, Govindan R. Lung cancer. In: Govindan R, Arquette MA, Lieber RL, eds. The Washington manual of oncology. Philadelphia: Lippincott, Williams and Wilkins; 2002:238–251.
Bass LA, McCarthy DW, Jones LA, et al. High purity production and potential applications of copper-60 and copper-61. J Labeled Compd Radiopharm 1997; 40:325–327.
McCarthy DW, Bass LA, Cutler PD, et al. High purity production and potential applications of copper-60 and copper-61. Nucl Med Biol 1999; 26:351–358.
Young H, Carnochan P, Zweit J, et al. Evaluation of copper(II)-pyruvaldehyde bis(N-4-methylthiosemicarbazone) for tissue blood flow using a trapped tracer model. J Nucl Med 1994; 21:336–341.
Adam L-E, Zaers J, Ostertag H, et al. Performance evaluation of the whole-body PET scanner ECAT EXAT HR+ following the IEC standard. IEEE Trans Nucl Sci 1997; 44:1172–1179.
Derenzo SE, Budinger TF, Juesman RH, Cahoon JL, Vuletich T. Imaging properties of a positron tomograph with 280 BGO crystals. IEEE Trans Nucl Sci 1981; 28:81–89.
Rydzewski B, Dehdashti F, Gordon BA, Teefey SA, Strasberg SM, Siegel BA. Usefulness of intraoperative sonography for revealing hepatic metastases from colorectal cancer in patients selected for surgery after undergoing FDG-PET. AJR 2002; 178:353–358.
Kubota K, Matsuzawa T, Ito M, et al. Lung tumor imaging by positron emission tomography using C-11 l-methionine. J Nucl Med 1985; 26:37–42.
World Health Organization. Handbook for Reporting Results of Cancer Treatment Geneva, Switzerland. World Health Organization, 1979; WHO Offset Publication No. 48.
Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun M. Cancer statistics, 2003. CA Cancer J Clin 2003; 53:5–26.
Fletcher GH, Lindberg RD, Caderao JB, et al. Hyperbaric oxygen as a radiotherapeutic adjuvant in advanced carcinoma of the uterine cervix: preliminary results of a randomized trial. Cancer 1977; 39:617–623.
Denny WA, Wilson WR. Tirapazamine: a bioreductive anticancer drug that exploits tumour hypoxia. Expert Opin Investig Drugs 2000; 9:2889–2901.
Kolstad P. Intercapillary distance, oxygen tension and local recurrence in cervix cancer. Scand J Clin Lab Invest Suppl 1968; 106:145–157.
Pappová N, Siracká E, Vacek A, Durkovsky J. Oxygen tension and prediction of the radiation response; polarographic study in human breast cancer. Neoplasma 1982; 29:669–674.
Gatenby RA, Kessler HB, Rosenblum JS et al. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys 1988; 14:831–838.
Vaupel P, Schlenger K, Knoop C, Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 1991; 51:3316–3322.
Höckel M, Schlenger K, Knoop C, Vaupel P. Oxygenation of carcinomas of the uterine cervix: evaluation by computerized O2 tension measurements. Cancer Res 1991; 51:6098–6102.
Höckel M, Knoop C, Schlenger K, Vorndran B, Knapstein PG, Vaupel P. Intra-tumor pO2 histography as predictive assay in advanced cancer of the uterine cervix. Adv Exp Med Biol 1994; 345:445–450.
Höckel M, Schlenger K, Höckel S, Aral B, Schäffer U, Vaupel P. Tumor hypoxia in pelvic recurrences of cervical cancer. Int J Cancer (Pred Oncol) 1998; 79:365–369.
Dearling JLJ, Lewis JS, McCarthy DW, Welch MJ, Blower PJ. Redox-active metal complexes for imaging hypoxic tissues: structure-activity relationships in copper(II) bis(thiosemicarbazone) complexes. Chem Commun 1998; 22:2531–2533.
Dearling JL, Lewis JS, Mullen GE, Welch MJ, Blower PJ. Copper bis(thiosemicarbazone) complexes as hypoxia imaging agents: structure-activity relationships. J Biol Inorganic Chem 2002; 7:249–259.
Takahashi N, Fujibayashi Y, Yonekura Y et al. Evaluation of62Cu labeled diacetyl-bis(N4-methylthiosemicarbazone) as a hypoxic tissue tracer in patients with lung cancer. Ann Nucl Med 2000; 14:323–328.
Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on18F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: an analysis of 125 cases. J Clin Oncol 1999; 17:3201–3206.
Ahuja V, Coleman RE, Herndon J, Patz EF. The prognostic significance of fluorodeoxyglucose positron emission tomography imaging for patients with non-small cell lung carcinoma. Cancer 1998; 83:918–924.
Oshida M, Uno K, Suzuki M, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer 1998; 82:2227–2234.
Burgman P, Odonoghue JA, Humm JL, Ling CC. Hypoxia-induced increase in FDG uptake in MCF7 cells. J Nucl Med 2001; 42:170–175.
Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Maziere B. FDG accumulation and tumor biology. Nucl Med Biol 1998; 25:317–322.
Dehdashti F, Grigsby PW, Mintun Mark A, Lewis J, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with60Cu-ATSM: relationship to therapeutic response. Int J Radiat Oncol Biol Phys 2003; 55:1233–1238.
McCarthy DW, Shefer RE, Klinkowstein RE, et al. Efficient production of high specific activity64Cu using a biomedical cyclotron. Nucl Med Biol 1997; 24:35–43.
Haynes NG, Lacy JL, Nayak N, et al. Performance of a62Zn/62Cu generator in clinical trials of PET perfusion agent 62Cu-PTSM. J Nucl Med 2000; 41:309–314.
Acknowledgements
This work was supported by NIH Grant CA81525 and DOE Grant DE-FG02-87ER60512.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dehdashti, F., Mintun, M.A., Lewis, J.S. et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging 30, 844–850 (2003). https://doi.org/10.1007/s00259-003-1130-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1130-4