Abstract
Over the years several analytical methods have been proposed for the measurement of glucose metabolism using fluorine-18 fluorodeoxyglucose ([18F]FDG) and positron emission tomography (PET). The purpose of this study was to evaluate which of these (often simplified) methods could potentially be used for clinical response monitoring studies in breast cancer. Prior to chemotherapy, dynamic [18F]FDG scans were performed in 20 women with locally advanced (n=10) or metastasised (n=10) breast cancer. Additional PET scans were acquired after 8 days (n=8), and after one, three and six courses of chemotherapy (n=18, 10 and 6, respectively). Non-linear regression (NLR) with the standard two tissue compartment model was used as the gold standard for measurement of [18F]FDG uptake and was compared with the following methods: Patlak graphical analysis, simplified kinetic method (SKM), SUV-based net influx constant ("Sadato" method), standard uptake value [normalised for weight, lean body mass (LBM) and body surface area (BSA), with and without corrections for glucose (g)], tumour to non-tumour ratio (TNT), 6P model and total lesion evaluation (TLE). Correlation coefficients between each analytical method and NLR were calculated using multilevel analysis. In addition, for the most promising methods (Patlak, SKM, SUVLBMg and SUVBSAg) it was explored whether correlation with NLR changed with different time points after the start of therapy. Three methods showed excellent correlation (r>0.95) with NLR for the baseline scan: Patlak10–60 and Patlak10–45 (r=0.98 and 0.97, respectively), SKM40–60 (r=0.96) and SUVLBMg (r=0.96). Good correlation was found between NLR and SUV-based net influx constant, TLE and SUVBSAg (0.90<r<0.95). The 6P model and TNT had the lowest correlation (r≤0.84). SUV was least accurate in predicting changes in [18F]FDG uptake over time during therapy. For all methods, correlation with NLR was significantly lower for bone metastases than for other (primary or metastatic) tumour lesions (P<0.05). In conclusion, three methods with different degrees of complexity appear to be promising alternatives to NLR for measuring glucose metabolism in breast cancer: Patlak, SKM and SUV (normalised for LBM and with a correction for plasma glucose).

Similar content being viewed by others
References
Kubota K, Matsuzawa T, Amemiya A, et al. Imaging of breast cancer with [18F]fluorodeoxyglucose and positron emission tomography. J Comput Assist Tomogr 1989; 13:1097–1098.
Wahl RL, Cody RL, Hutchins GD, et al. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-d-glucose. Radiology 1991; 179:765–770.
Tse NY, Hoh CK, Hawkins RA, et al. The application of positron emission tomographic imaging with fluorodeoxyglucose to the evaluation of breast disease. Ann Surg 1992; 216:27–34.
Avril N, Dose J, Janicke F, et al. Metabolic characterization of breast tumors with positron emission tomography using F-18 fluorodeoxyglucose. J Clin Oncol 1996; 14:1848–1857.
Noh DY, Yun IJ, Kim JS, et al. Diagnostic value of positron emission tomography for detecting breast cancer. World J Surg 1998; 22:223–227.
Avril N, Rose CA, Schelling M, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 2000; 18:3495–3502.
Czernin J. FDG-PET in breast cancer: a different view of its clinical usefulness [review]. Mol Imag Biol 2002; 4:35–45.
Nieweg OE, Kim EE, Wong WH, et al. Positron emission tomography with fluorine-18-deoxyglucose in the detection and staging of breast cancer. Cancer 1993; 71:3920–3925.
Adler LP, Crowe JP, al-Kaisi NK, et al. Evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2-fluoro-d-glucose PET. Radiology 1993; 187:743–750.
Utech CI, Young CS, Winter PF. Prospective evaluation of fluorine-18 fluorodeoxyclucose positron emission tomography in breast cancer for staging of the axilla related to surgery and immunocytochemistry. Eur J Nucl Med 1996; 23:1588–1593.
Avril N, Dose J, Janicke F, Ziegler S, et al. Assessment of axillary lymph node involvement in breast cancer patients with positron emission tomography using radiolabeled 2-(fluorine-18)-fluoro-2-deoxy-d-glucose. J Natl Cancer Inst 1996; 88:1204–1209.
Smith IC, Ogston KN, Whitford P, et al. Staging of the axilla in breast cancer: accurate in vivo assessment using positron emission tomography with 2-(fluorine-18)-fluoro-2-deoxy-d-glucose. Ann Surg 1998; 228:220–227.
Wahl RL, Zasadny K, Helvie M, et al. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol 1993; 11:2101–2111.
Bruce DM, Evans NT, Heys SD, et al. Positron emission tomography: 2-deoxy-2-[18F]-fluoro-d-glucose uptake in locally advanced breast cancers. Eur J Surg Oncol 1995; 21:280–283.
Jansson T, Westlin JE, Ahlstrom H, et al. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: a method for early therapy evaluation? J Clin Oncol 1995; 13:1470–1477.
Bassa P, Kim EE, Inoue T, et al. Evaluation of preoperative chemotherapy using PET with fluorine-18-fluorodeoxyglucose in breast cancer. J Nucl Med 1996; 37:931–938.
Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [(18)F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol 2000; 18:1689–1695.
Tiling R, Linke R, Untch M, et al.18F-FDG PET and 99mTc-sestamibi scintimammography for monitoring breast cancer response to neoadjuvant chemotherapy: a comparative study. Eur J Nucl Med 2001; 28:711–720.
van der Hiel B, Pauwels EK, Stokkel MP. Positron emission tomography with 2-[18F]-fluoro-2-deoxy-d-glucose in oncology. Part IIIa. Therapy response monitoring in breast cancer, lymphoma and gliomas [review]. J Cancer Res Clin Oncol 2001; 127:269–277.
Oshida M, Uno K, Suzuki M, et al. Predicting the prognoses of breast carcinoma patients with positron emission tomography using 2-deoxy-2-fluoro[18F]-d-glucose. Cancer 1998; 82:2227–2234.
Smith IC, Welch AE, Hutcheon AW, et al. Positron emission tomography using [(18)F]-fluorodeoxy-d-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000; 18:1676–1688.
Sokoloff L, Reivich M, Kennedy C, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 1977; 28:897–916.
Phelps ME, Huang SC, Hoffman EJ. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-d-glucose: validation of method. Ann Neurol 1979; 6:371–388.
Huang SC, Phelps ME, Hoffman EJ. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol 1980; 238:E69–E82.
Keyes JWJ. SUV: standard uptake or silly useless value? J Nucl Med 1995; 36:1836–1839.
Hamberg LM, Hunter GJ, Alpert NM, et al. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med 1994; 35:1308–1312.
Huang SC. Anatomy of SUV. Nucl Med Biol 2000; 27:643–646.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab 1983; 3:1–7.
Hunter GJ, Hamberg LM, Alpert NM, Choi NC, Fischman AJ. Simplified measurement of deoxyglucose utilization rate. J Nucl Med 1996; 37:950–955.
Sadato N, Tsuchida T, Nakaumra S, et al. Non-invasive estimation of the net influx constant using the standardized uptake value for quantification of FDG uptake of tumours. Eur J Nucl Med 1998; 25:559–564.
Imran MB, Kubota K, Yamada S, et al. Lesion-to-background ratio in nonattenuation-corrected whole-body FDG PET images. J Nucl Med 1998; 39:1219–1223.
Wu HM, Hoh CK, Huang SC, Yao WJ, Phelps ME, Hawkins RA. Quantification of serial tumor glucose metabolism. J Nucl Med 1996; 37:506–513.
Wu HM, Huang SC, Choi Y, Hoh CK, Hawkins RA. A modeling method to improve quantitation of fluorodeoxyglucose uptake in heterogeneous tumor tissue. J Nucl Med 1995; 36:297–306.
Young H, Baum R, Cremerius U, et al. Measurement of clinical and sub-clinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. Eur J Cancer 1999; 35:1773–1782.
Brix G, Zaers J, Adam LE, et al. Performance evaluation of a whole-body PET scanner using the NEMA protocol. National Electrical Manufacturers Association. J Nucl Med 1997; 38:1614–1623.
Hudson HM, Larkin RS. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans Med Imaging 1994; 13:601–609.
Boellaard R, van Lingen A, Lammertsma AA. Experimental and clinical evaluation of iterative reconstruction (OSEM) in dynamic PET: quantitative characteristics and effects on kinetic modeling. J Nucl Med 2001; 42:808–817.
Hoekstra C, Hoekstra O, Lammertsma AA. On the use of image derived input functions in oncological FDG PET studies. Eur J Nucl Med 1999; 26:1489–1492.
van der Weerdt AP, Klein LJ, Boellaard R, et al. Image-derived input functions for determination of MRglu in cardiac18F-FDG PET scans. J Nucl Med 2001; 42:1622–1629.
Hawkins RA, Phelps ME, Huang SC, et al. A kinetic evaluation of blood-brain barrier permeability in human brain tumors with [68Ga]EDTA and positron computed tomography. J Cereb Blood Flow Metab 1984; 4:507–515.
Akaike H. A new look at the statistical identification. IEEE Trans Automat Contr 1978; 19:716–723.
Schwarz G. Estimating the dimension of a model. Ann Statist 1978; 6:461–464.
Hoekstra C, Paglianiti I, Hoekstra O, et al. Monitoring response to therapy in cancer using [18F]-2-fluoro-2-deoxy-d-glucose and positron emission tomography: an overview of different analytical methods. Eur J Nucl Med 2000; 27:731–743.
Goldstein, H. Multilevel statistical models. London: Edward Arnold, 1995.
Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med 1994; 35:164–167.
Kim CK, Gupta NC. Dependency of standardized uptake values of fluorine-18 fluorodeoxyglucose on body size: comparison of body surface area correction and lean body mass correction. Nucl Med Commun 1996; 17:890–894.
Schomburg A, Bender H, Reichel C, et al. Standardized uptake values of fluorine-18 fluorodeoxyglucose: the value of different normalization procedures. Eur J Nucl Med 1996; 23:571–574.
Sugawara Y, Zasadny KR, Neuhoff AW, et al. Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology 1999; 213:521–525.
Hoekstra CJ, Hoekstra OS, Stroobants SG, et al. Methods to monitor response to chemotherapy in non-small cell lung cancer with18F-FDG PET. J Nucl Med 2002; 43:1304–1309.
Avril N, Bense S, Ziegler SI, et al. Breast imaging with fluorine-18-FDG PET: quantitative image analysis. J Nucl Med 1997; 38:1186–1191.
Kole AC, Nieweg OE, Pruim J, et al. Standardized uptake value and quantification of metabolism for breast cancer imaging with FDG andl-[1-11C]tyrosine PET. J Nucl Med 1997; 38:692–696.
Graham MM, Peterson LM, Hayward RM. Comparison of simplified quantitative analyses of FDG uptake. Nucl Med Biol 2000; 7:647–655.
Langen KJ, Braun U, Rota KE, et al. The influence of plasma glucose levels on fluorine-18- fluorodeoxyglucose uptake in bronchial carcinomas. J Nucl Med 1993; 34:355–359.
Lindholm P, Minn H, Leskinen-Kallio S, et al. Influence of the blood glucose concentration on FDG uptake in cancer-a PET study. J Nucl Med 1993; 34:1–6.
Zasadny KR, Wahl RL. Enhanced FDG-PET tumor imaging with correlation-coefficient filtered influx-constant images. J Nucl Med 1996; 37:371–374.
Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-d-glucose: variations with body weight and a method for correction. Radiology 1993; 189:847–850.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
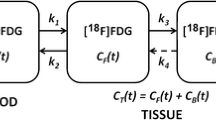
Patlak graphical analysis (Patlak)
This method [28] assumes unidirectional uptake of [18F]FDG (i.e. k 4=0), with irreversible trapping in tissue as [18F]FDG-6-PO4. The Patlak method was applied to several data intervals: 10–60, 10–45, 10–30, 20–60 and 30–60 min post injection. The lumped constant LC was assumed to be unity.
Standard uptake value (SUV)
The SUV is the ratio of tissue concentration and injected activity [25, 26, 27]. It is a semi-quantitative method, which does not take FDG kinetics into account. In the present study calculations were performed using both 40–60 and 50–60 min acquisition intervals. SUV was normalised to weight (SUVW), lean body mass (SUVLBM) [56] and body surface area (SUVBSA) [45] and calculated with and without plasma glucose (g) correction. BSA=0.007184×weight0.425×height0.725 and LBM=45.5+0.91(height−152).
The simplified kinetic method (SKM)
The SKM [29] is similar to the SUV (static image with both 40–60 and 50–60 min acquisition intervals), except that a correction is made for the actual plasma clearance of [18F]FDG. In the present analysis, venous blood samples taken at 35, 45 and 55 min post injection were used to incorporate the individual plasma clearance.
The SUV-based net influx constant ("Sadato" method)
In this method [30] the net influx constant (K i) is derived from SUV and a population-based plasma input function. This analysis was performed for the 40–60 min acquisition interval and corrections for both body weight and body surface area were applied.
Two-ROI, 6P method (6P model)
The 6P model [33] combines the kinetics in two ROIs (tumour and non-tumour reference regions) using six parameters (four rate constants, tumour and vascular fractions). Like NLR, it requires full dynamic scanning, but it accounts for the admixture of non-tumour components in a tumour. In this paper either small (6P modelsmall) or large (6P modellarge) reference regions in the normal breast were used.
Tumour to non-tumour ratio (TNT)
The TNT is a simple semi-quantitative index that only requires a static image and no scanner calibration. In this study, TNT was measured using 40–60 and 50–60 min acquisition intervals. As non-tumour regions, the same ROIs were used as for the 6P model.
Total lesion evaluation (TLE)
Correlation coefficient constrained parametric images (10–60 min acquisition interval) were obtained from the Patlak parametric images by setting all pixels with a linear correlation coefficient below a certain threshold to 0 [32]. Correlation coefficient thresholds of 0.5, 0.6, 0.7, 0.8 and 0.9 were used. All images containing a tumour lesion were summed, a single ROI was defined covering the entire lesion in this summed image, and subsequently the total lesion metabolic index was calculated.
Rights and permissions
About this article
Cite this article
Krak, N.C., van der Hoeven, J.J.M., Hoekstra, O.S. et al. Measuring [18F]FDG uptake in breast cancer during chemotherapy: comparison of analytical methods. Eur J Nucl Med Mol Imaging 30, 674–681 (2003). https://doi.org/10.1007/s00259-003-1127-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1127-z