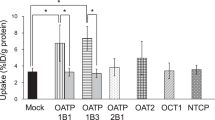
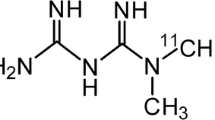
Abstract
Technetium-99m sestamibi has attracted interest for assessment of the function of P-glycoproteins, which are well expressed in the liver and have roles in biliary transport and the removal of chemotherapeutic drugs. To further examine the cross-reactivity of 99mTc-sestamibi for P-glycoprotein family members, we conducted studies in animals. Hepatobiliary secretion of 99mTc-sestamibi was determined in normal FVB/N mice, mutant mice with specific P-glycoprotein deficiencies in the FVB/N background, normal Long-Evans Agouti (LEA) rats, and Long-Evans Cinnamon (LEC) rats with abnormal copper transport and liver disease but intact P-glycoprotein expression. After intrasplenic injection, 99mTc-sestamibi was rapidly incorporated in the mouse and rat liver, with maximal accumulation after 102±31 and 109±16 s, respectively (P=NS). In normal mice and rats, 55%±11% and 55%±6%, respectively, of the maximal sestamibi activity was retained in the liver after 1 h (P=NS). In double knockout mice lacking both mdr1a and mdr1b homologs of the human MDR1 (ABCB1) gene, 88%±11% of maximal sestamibi activity was retained in the liver after 1 h (P<0.001). In knockout mice deficient in either mdr1a gene or mdr2 (ABCB4) gene, biliary sestamibi excretion was also impaired, although this impairment was relatively less pronounced in ABCB4-deficient mice than in double knockout mice lacking both ABCB1 gene homologs (P<0.03). Hepatobiliary sestamibi excretion in LEC rats was not different from that in control normal rats, despite the presence of significant liver disease in the former. Hepatobiliary sestamibi excretion requires P-glycoproteins and is unperturbed in chronic liver disease. Sestamibi appears to be a substrate for both ABCB1 and ABCB4 genes, although the former utilizes it far more efficiently. Assessment of P-glycoprotein activity with sestamibi should consider how regulation of ABCB1 and related family members might modulate sestamibi incorporation.




Similar content being viewed by others
References
Jain D. Technetium-99m labeled myocardial perfusion imaging agent. Semin Nucl Med 1999; 29:221–236.
Mobasseri S, Hendel RC. Cardiac imaging in women: use of radionuclide myocardial perfusion imaging and echocardiography for acute chest pain. Cardiol Rev 2002; 10:149–160.
Chen CC, Meadows B, Regis J, Kalafsky G, Fojo T, Carrasquillo JA, Bates SE. Detection of in vivo P-glycoprotein inhibition by PSC 833 using Tc-99m sestamibi. Clin Cancer Res 1997; 3:545–552.
Luker GD, Fracasso PM, Dobkin J, Piwnica-Worms D. Modulation of the multidrug resistance P-glycoprotein: detection with technetium-99m-sestamibi in vivo. J Nucl Med 1997; 38:369–372.
Chen WS, Luker KE, Dahlheimer JL, Pica CM, Luker GD, Piwnica-Worms D. Effects of MDR1 and MDR3 P-glycoproteins, MRP1, and BCRP/MXR/ABCP on the transport of (99m)Tc-tetrofosmin. Biochem Pharmacol 2000; 60:413–426.
Perek N, Koumanov F, Denoyer D, Boudard D, Dubois F. Modulation of the multidrug resistance of glioma by glutathione levels depletion--interaction with Tc-99m-sestamibi and Tc-99m-tetrofosmin. Cancer Biother Radiopharm 2002; 17:291–302.
Derebek E, Capa G, Berk H, Sekeroglu B, Havitcioglu H, Degirmenci B, Alakavuklar M, Durak H. Tc-99m sestamibi imaging as an indicator of P-glycoprotein expression in metastatic pheochromocytoma. Clin Nucl Med 1998; 23:637–638.
Ciarmiello A, Del Vecchio S, Silvestro P, Potena MI, Carriero MV, Thomas R, Botti G, D'Aiuto G, Salvatore M. Tumor clearance of technetium 99m-sestamibi as a predictor of response to neoadjuvant chemotherapy for locally advanced breast cancer. J Clin Oncol 1998; 16:1677–1683.
Kim YS, Cho SW, Lee KJ, et al. Tc-99m MIBI SPECT is useful for noninvasively predicting the presence of MDR1 gene-encoded P-glycoprotein in patients with hepatocellular carcinoma. Clin Nucl Med 1999; 24:874–879.
Mubashar M, Harrington KJ, Chaudhary KS, Lalani el-N, Stamp GW, Sinnett D, Glass DM, Peters AM.99mTc-sestamibi imaging in the assessment of toremifene as a modulator of multidrug resistance in patients with breast cancer. J Nucl Med 2002; 43:519–525.
Dirlik A, Burak Z, Goksel T, Erinc R, Karakus H, Ozcan Z, Veral A, Ozhan M. The role of Tc-99m sestamibi imaging in predicting clinical response to chemotherapy in lung cancer. Ann Nucl Med 2002; 16:103–108.
Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2002; 2:48–58.
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 1999; 39:361–398.
Kerb R, Hoffmeyer S, Brinkmann U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics 2001; 2:51–64.
Labialle S, Gayet L, Marthinet E, Rigal D, Baggetto LG. Transcriptional regulators of the human multidrug resistance 1 gene: recent views. Biochem Pharmacol 2002; 64:943–948.
Klein I, Sarkadi B, Varadi A. An inventory of the human ABC proteins. Biochim Biophys Acta 1999; 1461:237–262.
Banker DE, Cooper JJ, Fennell DA, Willman CL, Appelbaum FR, Cotter FE. PK11195, a peripheral benzodiazepine receptor ligand, chemosensitizes acute myeloid leukemia cells to relevant therapeutic agents by more than one mechanism. Leuk Res 2002; 26:91–106.
Schiedlmeier B, Schilz AJ, Kuhlcke K, Laufs S, Baum C, Zeller WJ, Eckert HG, Fruehauf S. Multidrug resistance 1 gene transfer can confer chemoprotection to human peripheral blood progenitor cells engrafted in immunodeficient mice. Hum Gene Ther 2002; 13:233–242.
Borst P, Evers R, Kool M, Wijnholds J. A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 2000; 92:1295–1302.
Yang Y, Chen Q, Zhang JT. Structural and functional consequences of mutating cysteine residues in the amino terminus of human multidrug resistance-associated protein 1. J Biol Chem 2002; 277:44268–44277.
Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell 2000; 103:757–768.
Hashimoto K, Uchiumi T, Konno T, Ebihara T, Nakamura T, Wada M, Sakisaka S, Maniwa F, Amachi T, Ueda K, Kuwano M. Trafficking and functional defects by mutations of the ATP-binding domains in MRP2 in patients with Dubin-Johnson syndrome. Hepatology 2002; 36:1236–1245.
Schinkel AH, Mayer U, Wagenaar E, et al. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A 1997; 94:4028–4033.
Smit JJ, Schinkel AH, Oude Elferink RP, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993; 75:451–462.
De Vree JM, Ottenhoff R, Bosma PJ, Smith AJ, Aten J, Oude Elferink RP. Correction of liver disease by hepatocyte transplantation in a mouse model of progressive familial intrahepatic cholestasis. Gastroenterology 2000; 119:1720–1730.
Terada K, Sugiyama T. The Long-Evans Cinnamon rat: an animal model for Wilson's disease. Pediatr Int 1999; 41:414–418.
Schilsky ML, Quintana N, Volenberg I, Kabishcher V, Sternlieb I. Spontaneous cholangiofibrosis in Long-Evans Cinnamon rats: a rodent model for Wilson's disease. Lab Animal Sci 1998; 48:156–161.
Schilsky M, Stockert R, Sternlieb I. Copper metabolism in the LEC rat, a rodent model of Wilson's disease. Am J Physiol 1994; 266:G907–G913.
Schilsky ML, Irani AN, Gorla GR, Volenberg I, Gupta S. Biliary copper excretion capacity in intact animals: correlation between ATP7B gene function, hepatic mass and copper excretion. J Biochem Mol Toxicol 2000; 14:210–214.
Malhi H, Irani AN, Volenberg I, Schilsky ML, Gupta S. Early cell transplantation in LEC rats modeling Wilson's disease eliminates hepatic copper with reversal of liver disease. Gastroenterology 2002; 122:438–447.
Gupta S, Lee C-D, Vemuru RP, Bhargava KK.111Indium-labeling of hepatocytes for analyzing biodistribution of transplanted cells. Hepatology 1994; 19:750–757.
Gupta S, Vasa SRG, Rajvanshi P, Zuckier L, Palestro CJ, Bhargava KK. Analysis of hepatocyte distribution and survival in vascular beds with cells marked by 99m-Tc or endogenous dipeptidyl peptidase IV activity. Cell Transpl 1997; 6:377–386.
Gottesman MM, Pastan I. The multidrug-transporter: a double-edged sword. J Biol Chem 1988; 263:12163–12166.
Roepe PD. The role of the MDR protein in altered drug translocation across tumor cell membranes. Biochim Biophys Acta 1995; 1241:385–405.
Wadkins RM, Roepe PD. Biophysical aspects of P-glycoprotein-mediated multidrug resistance. Int Rev Cytol 1997; 171:121–165.
Barbarics E, Kronauge JF, Davison A, Jones AG. Uptake of cationic technetium complexes in cultured human carcinoma cells and human xenografts. Nucl Med Biol 1998; 25:667–673.
Arbab AS, Koizumi K, Toyama K, Arai T, Araki T. Effects of ion channel modulators in the influx and efflux of Tc-99m-MIBI. Ann Nucl Med 1999; 13:27–32.
Sugawara I, Kataoka I, Morishita Y, et al. Tissue distribution of P-glycoprotein encoded by a multidrug-resistance gene as revealed by a monoclonal antibody MRK 16. Cancer Res 1988; 48:1926–1929.
Cordon-Cardo C, O'Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci U S A 1989; 86:695–698.
Brady JM, Cherrington NJ, Hartley DP, Buist SC, Li N, Klaassen CD. Tissue distribution and chemical induction of multiple drug resistance genes in rats. Drug Metab Dispos 2002; 30:838–844.
Smit JJ, Schinkel AH, Mol CA, et al. Tissue distribution of the human MDR3 P-glycoprotein. Lab Invest 1994; 71:638–649.
Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A 1999; 96:3900–3905.
Vos TA, Ros JE, Havinga R, Moshage H, Kuipers F, Jansen PL, Muller M. Regulation of hepatic transport systems involved in bile secretion during liver regeneration in rats. Hepatology 1999; 29:1833–1839.
Lecureur V, Thottassery JV, Sun D, Schuetz EG, Lahti J, Zambetti GP, Schuetz JD. Mdr1b facilitates p53-mediated cell death and p53 is required for Mdr1b upregulation in vivo. Oncogene 2001; 20:303–313.
Zampieri L, Bianchi P, Ruff P, Arbuthnot P. Differential modulation by estradiol of P-glycoprotein drug resistance protein expression in cultured MCF7 and T47D breast cancer cells. Anticancer Res 2002; 22:2253–2259.
Kato J, Kobune M, Kohgo Y, et al. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long-Evans Cinnamon rats. J Clin Invest 1996; 98:923–929.
Schaefer M, Hopkins RG, Failla ML, Gitlin JD. Hepatocyte-specific localization and copper-dependent trafficking of the Wilson's disease protein in the liver. Am J Physiol 1999; 276:G639–G646.
Naddaf SY, Akisik MF, Aziz M, Omar WS, Hirschfeld A, Masdeu J, Donnenfeld H, Abdel-Dayem HM. Comparison between201Tl-chloride and 99Tc(m)-sestamibi SPET brain imaging for differentiating intracranial lymphoma from non-malignant lesions in AIDS patients. Nucl Med Commun 1998; 19:47–53.
Fonti R, Del Vecchio S, Zannetti A, De Renzo A, Di Gennaro F, Catalano L, Califano C, Pace L, Rotoli B, Salvatore M. Bone marrow uptake of99mTc-MIBI in patients with multiple myeloma. Eur J Nucl Med 2001; 28:214–220.
Carpinteiro A, Peinert S, Ostertag W, Zander AR, Hossfeld DK, Kuhlcke K, Eckert HG, Baum C, Hegewisch-Becker S. Genetic protection of repopulating hematopoietic cells with an improved MDR1-retrovirus allows administration of intensified chemotherapy following stem cell transplantation in mice. Int J Cancer 2002; 98:785–792.
Bunting KD. ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 2002; 20:11–20.
Acknowledgements
We thank Dr. J. Kandimalla for assistance with animal surgery, and our late colleague, Dr. Menes Afriyie, for image analysis. The work was supported in part by NIH grants RO1 DK46952, P30 DK41296, P30 CA13330, and MO1 RR12248, and by grant 3894 from the Long Island Jewish Medical Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joseph, B., Bhargava, K.K., Malhi, H. et al. Sestamibi is a substrate for MDR1 and MDR2 P-glycoprotein genes. Eur J Nucl Med Mol Imaging 30, 1024–1031 (2003). https://doi.org/10.1007/s00259-002-1111-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-002-1111-z