Abstract
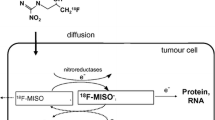
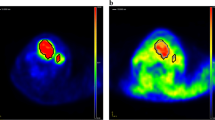
Hypoxia imparts resistance to radiotherapy and chemotherapy and also promotes a variety of changes in tumor biology through inducible promoters. The purpose of this study was to evaluate the use of positron emission tomography (PET) imaging with fluorine-18 fluoromisonidazole (FMISO) in soft tissue sarcomas (STS) as a measure of hypoxia and to compare the results with those obtained using [18F]fluorodeoxyglucose (FDG) and other known biologic correlates. FDG evaluates energy metabolism in tumors while FMISO uptake is proportional to tissue hypoxia. FMISO uptake was compared with FDG uptake. Vascular endothelial growth factor (VEGF) expression was also compared with FMISO uptake. Nineteen patients with STS underwent PET scanning with quantitative determination of FMISO and FDG uptake prior to therapy (neo-adjuvant chemotherapy or surgery alone). Ten patients receiving neo-adjuvant chemotherapy were also imaged after chemotherapy but prior to surgical resection. Standardized uptake value (SUV) was used to describe FDG uptake; regional tissue to blood ratio (≥1.2 was considered significant) was used for FMISO uptake. Significant hypoxia was found in 76% of tumors imaged prior to therapy. No correlation was identified between pretherapy hypoxic volume (HV) and tumor grade (r=0.15) or tumor volume (r=0.03). The correlation of HV with VEGF expression was 0.39. Individual tumors showed marked heterogeneity in regional VEGF expression. The mean pixel-by-pixel correlation between FMISO and FDG uptake was 0.49 (range 0.09–0.79) pretreatment and 0.32 (range −0.46–0.72) after treatment. Most tumors showed evidence of reduced uptake of both FMISO and FDG following chemotherapy. FMISO PET demonstrates areas of significant and heterogeneous hypoxia in soft tissue sarcomas. The significant discrepancy between FDG and FMISO uptake seen in this study indicates that regional hypoxia and glucose metabolism do not always correlate. Similarly, we did not find any relationship between the hypoxic volume and the tumor volume or VEGF expression. Identification of hypoxia and development of a more complete biologic profile of STS will serve to guide more rational, individualized cancer treatment approaches.







Similar content being viewed by others
References
Mazanet R, Antman KH. Sarcomas of soft tissue and bone. Cancer 1991; 68:463–473.
Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev 1994; 13:139–168.
Sanna K, Rofstad EK. Hypoxia-induced resistance to doxorubicin and methotrexate in human melanoma cell lines in vitro. Int J Cancer 1994; 58:258–262.
Sakata K, Kwok TT, Murphy BJ, Laderoute KR, Gordon GR, Sutherland RM. Hypoxia-induced drug resistance: comparison to P-glycoprotein-associated drug resistance. Br J Cancer 1991; 64:809–814.
Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A 1988; 85:9533–9537.
Stackpole, CW. Intrapulmonary spread of established B16 melanoma lung metastases and lung colonies. Invasion Metastasis 1990; 10:267–280.
Hill RP. Tumor progression: potential role of unstable genomic changes. Cancer Metastasis Rev 1990; 9:137–147.
Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol 2000; 76:589–605.
Rasey JS, Hofstrand PD, Chin LK, Tewson TJ. Characterization of [18F]fluoroetanidazole, a new radiopharmaceutical for detecting tumor hypoxia. J Nucl Med 1999; 40:1072–1079.
Brown MJ. The hypoxic cell: a target for selective cancer therapy—Eighteenth Bruce F. Cain memorial award lecture. Cancer Res 1999; 59:5863–5870.
Hockel M, Knoop C, Schlenger K, Vorndran B, Knapstein PG, Vaupel P. Intratumoral pO2 histography as predictive assay in advanced cancer of the uterine cervix. Adv Exp Med Biol 1994; 345:445–450.
Wijffels KI, Kaanders JH, Rijken PF, Bussink J, van den Hoogen FJ, Marres HA, de Wilde PC, Raleigh JA, van der Kogel AJ. Vascular architecture and hypoxic profiles in human head and neck squamous cell carcinomas. Br J Cancer 2000; 83:674–683.
Jiang BH, Semenza GL, Bauer C, Marti HH. Hypoxia-inducible factor 1 levels vary exponentially over a physiologically relevant range of O2 tension. Am J Physiol 1996; 271:C1172–C1180.
Guillemin K, Krasnoq MA. The hypoxic response: huffing and HIFing. Cell 1997; 89:9–12.
Airley R, Loncaster J, Davidson S, Bromley M, Roberts S, Patterson A, Hunter R, Stratford I, West C. Glucose transporter glut-1 expression correlates with tumor hypoxia and predicts metastasis-free survival in advanced carcinoma of the cervix. Clin Cancer Res 2001; 7:928–934.
Jones DP, Tak Yee AW, Bai C, Sillau AH. Regulation of mitochondrial distribution: an adaptive response to changes in oxygen supply. In: Lahiri S, Cherniack NS, Fitzgerald RS, eds. Response and adaptation to hypoxia: organ to organelle. New York: Oxford University Press; 1991:25–35.
Brizel DM, Schroeder T, Scher RL, Walenta S, Clough RW, Dewhirst MW, Mueller-Klieser W. Elevated tumor lactate concentrations predict for an increased risk of metastases in head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001; 51:349–353.
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, Mueller-Klieser W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res 2000; 60:916–921.
Walenta S, Salameh A, Lyng H, Evensen JF, Mitze M, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in head and neck tumors with incidence of metastasis. Am J Pathol 1997; 150:409–415.
Star-Lack JM, Adalsteinsson E, Adam MF, Terris DJ, Pinto HA, Brown JM, Spielman DM. In vivo1H MR spectroscopy of human head and neck lymph node metastasis and comparison with oxygen tension measurements. AJNR Am J Neuroradiol 2000; 21:183–193.
Evans SM, Hahn SM, Magarelli DP, Koch CJ. Hypoxic heterogeneity in human tumors: EF5 binding, vasculature, necrosis, and proliferation. Am J Clin Oncol 2001; 24:467–472.
Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem 1996; 271:32253–32259.
Zhong H, Simons JW. Direct comparison of GAPDH, beta-actin, cyclophilin, and 28S rRNA as internal standards for quantifying RNA levels under hypoxia. Biochem Biophys Res Commun 1999; 259:523–526.
Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992; 359:843–845.
Helfman T, Falanga V. Gene expression in low oxygen tension. Am J Med Sci 1993; 306:37–41.
Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1:27–31.
Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001; 85:881–890.
Samoto K, Ikezaki K, Ono M, Shono T, Kohno K, Kuwano M, Fukui M. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res 1995; 55:1189–1193.
Toi M, Hoshina S, Takayanagi T, Tominaga T. Association of vascular endothelial growth factor expression with tumor angiogenesis and with early relapse in primary breast cancer. Jpn J Cancer Res 1994; 85:1045–1049.
Berger DP, Herbstritt L, Dengler WA, Marme D, Mertelsmann R, Fiebig HH. Vascular endothelial growth factor (VEGF) mRNA expression in human tumor models of different histologies. Ann Oncol 1995; 6:817–825.
Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Sowa M. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer 1996; 77:858–863.
Mattern J, Koomagi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid lung carcinoma. Br J Cancer 1996; 73:931–934.
Takahashi Y, Kitadai Y, Bucana CD, Cleary KR, Ellis LM. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res 1995; 55:3964–3968.
Brizel DM, Rosner GL, Prosnitz LR, Dewhirst MW. Patterns and variability of tumor oxygenation in human soft tissue sarcomas, cervical carcinomas, and lymph node metastases. Int J Radiat Oncol Biol Phys 1995; 32:1121–1125.
Chapman JD, Baer K, Lee J. Characteristics of the metabolism-induced binding of misonidazole to hypoxic mammalian cells. Cancer Res 1983; 43:1523–1528.
Chapman JD, Lee J, Meeker BE. Keynote address: cellular reduction of nitroimidazole drugs: potential for selective chemotherapy and diagnosis of hypoxic cells. Int J Radiat Oncol Biol Phys 1989; 16:911–917.
Grierson JR, Link JM, Mathis CA, Rasey JS, Krohn KA. A radiosynthesis of fluorine-18 fluoromisonidazole. J Nucl Med 1989; 30:343–350.
Rasey JS, Koh WJ, Evans ML, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Biol Phys 1996; 36:417–428.
Koh WJ, Rasey JS, Evans ML, Grierson JR, Lewellen TK, Graham MM, Krohn KA, Griffin TW. Imaging of hypoxia in human tumors with [F-18]fluoromisonidazole. Int J Radiat Oncol Biol Phys 1992; 22:199–212.
Casciari JJ, Graham MM, Rasey JS. A modeling approach for quantifying tumor hypoxia with [F-18]fluoromisonidazole PET time-activity data. Med Phys 1995; 22:1127–1139.
Costa J, Wesley RA, Glatstein E, Rosenberg SA. The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer 1984; 53:530–541.
Lewellen TK, Kohlmyer S, Miyaoka R, Schubert S, Stearns C. Investigation of the count rate performance of the General Electric Advance Positron Emission Tomograph. IEEE Trans Nucl Sci 1995; 42:1051–1057.
DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner. J Nucl Med 1994; 35:1398–1406.
Minoshima S, Berger KL, Lee KS, Mintun MA. An automated method for rotational correction and centering of three-dimensional functional brain images. J Nucl Med 1992; 33:1579–1585.
Brizel DM, Rosner GL, Harrelson J, Prosnitz LR, Dewhirst MW. Pretreatment oxygenation profiles of human soft tissue sarcomas. Int J Radiat Oncol Biol Phys 1994; 30:635–642.
Nordsmark M, Bentzen SM, Overgaard J. Measurement of human tumour oxygenation status by a polarographic needle electrode. An analysis of inter- and intratumour heterogeneity. Acta Oncol 1994; 33:383–389.
Rasey JS, Nelson NJ, Chin L, Evans ML, Grunbaum Z. Characteristics of the binding of labeled fluoromisonidazole in cells in vitro. Radiat Res 1990; 122:301–308.
Rasey JS, Martin GV, Krohn KA. Quantifying hypoxia with radiolabeled fluoromisonidazole: pre-clinical and clinical studies. In: Machulla HJ, ed. Imaging of hypoxia: tracer developments. Dordrecht: Kluwer Academic, 1999.
Adams GE, Barnes DW, du Boulay C, et al. Induction of hypoxia in normal and malignant tissues by changing the oxygen affinity of hemoglobin—implications for therapy. Int J Radiat Oncol Biol Phys 1986; 12:1299–1302.
Thomlinson RH, Gray LH. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer 1955; 9:539–549.
Moulder JE, Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev 1987; 5:313–341.
Vaupel P, Okunieff P, Kallinowski F, Neuringer LJ. Correlations between31P-NMR spectroscopy and tissue O2 tension measurements in a murine fibrosarcoma. Radiat Res 1989; 120:477–493.
Rasey JS, Koh WJ, Grierson JR, Grunbaum Z, Krohn KA. Radiolabelled fluoromisonidazole as an imaging agent for tumor hypoxia. Int J Radiat Oncol Biol Phys 1989; 17:985–991.
Nieweg OE, Pruim J, van Ginkel RJ, Hoekstra HJ, Paans AM, Molenaar WM, Koops HS, Vaalburg W. Fluorine-18-fluorodeoxyglucose PET imaging of soft-tissue sarcoma. J Nucl Med 1996; 37:257–261.
Eary JF, Mankoff DA. Tumor metabolic rates in sarcoma using FDG PET. J Nucl Med 1998; 39:250–254.
Eary JF, Conrad EU, Bruckner JD, Folpe A, Hunt KJ, Mankoff DA, Howlett AT. Quantitative [F-18]fluorodeoxyglucose positron emission tomography in pretreatment and grading of sarcoma. Clin Cancer Res 1998; 4:1215–1220.
Plate KH, Breier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer 1994; 59:520–529.
Acknowledgements
The authors wish to acknowledge the valuable help and useful suggestions provided by Mr. Mark Muzi, Research Scientist in the Department of Radiology regarding analysis of the data.
This research was supported by NIH grants CA-42045, CA-34570, and CA-65537
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajendran, J.G., Wilson, D.C., Conrad, E.U. et al. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging 30, 695–704 (2003). https://doi.org/10.1007/s00259-002-1096-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-002-1096-7