Abstract.
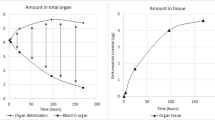
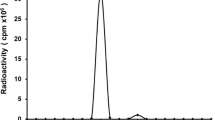
The native chimeric human-mouse anti-CD20 antibody IDEC-C2B8 (rituximab) is therapeutically applied in relapsed non-Hodgkin's lymphoma (NHL). The purpose of this study was to evaluate the distribution and pharmacokinetics of iodine-131 labelled rituximab in humans for radioimmunotherapy of relapsed CD20-positive NHL. Thirty-five patients with relapsed NHL were administered 20–40 mg rituximab labelled with 250 MBq 131I. Biodistribution was determined by the gamma camera whole-body scans, whole-body probe measurements and the analysis of serial blood and urine samples. Dosimetry was performed using the MIRDOSE 3 program. Antibody administration was well tolerated. The whole-body activity showed a mono-exponential decrease with a wide range of effective half-lives, the mean value (88 h) being significantly longer than the half-life of its murine counterpart, tositumomab. This led to appropriately higher dose factors for the whole body and organs. Activity was excreted mainly through the kidneys. Normal organs showed decreasing ratios of organ to whole-body activity over time, whereas the tumour tissue presented different kinetics, with increasing ratios of tumour to whole-body activity as evidence for specific antibody binding. It is concluded that 131I-labelled rituximab is suitable for pretherapeutic dosimetry. Due to the wide range of whole-body and organ dose factors, individual dosimetry is necessary for radioimmunotherapy with 131I-labelled rituximab. The therapeutic activities of 131I-labelled rituximab required to deliver similar doses should be lower than those of its murine counterpart.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received 19 October 2001 and in revised form 24 February 2002
Electronic Publication
Rights and permissions
About this article
Cite this article
Scheidhauer, K., Wolf, I., Baumgartl, HJ. et al. Biodistribution and kinetics of 131I-labelled anti-CD20 MAB IDEC-C2B8 (rituximab) in relapsed non-Hodgkin's lymphoma. Eur J Nucl Med 29, 1276–1282 (2002). https://doi.org/10.1007/s00259-002-0820-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-002-0820-7