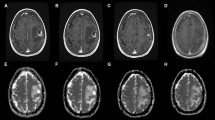
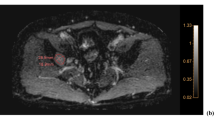
Abstract
Objective
To investigate the effect of sclerosis on apparent diffusion coefficient measurements in bone metastases from prostate cancer undergoing treatment.
Materials and methods
Sixteen patients underwent CT scans and MRI at baseline and 12 weeks following commencement of chemotherapy. For each patient, up to five bone metastases were selected. Hounsfield units were measured on CT and apparent diffusion coefficient (ADC) was measured on diffusion weighted MRI at both time points. Correlations between changes in apparent diffusion coefficient and Hounsfield units were investigated.
Results
Corresponding pre- and post-treatment apparent diffusion coefficient and Hounsfield units were available on 60 lesions from 16 patients. Overall, there was no significant correlation between changes in apparent diffusion coefficient with Hounsfield units. However, where changes in Hounsfield units increased by more than 50 %, there was a trend for an associated ADC rise.
Conclusions
Increasing sclerosis of bone metastases on treatment does not significantly impede diffusion.



Similar content being viewed by others
References
Costelloe CM, Rohren EM, Madewell JE, et al. Imaging bone metastases in breast cancer: techniques and recommendations for diagnosis. Lancet Oncol. 2009;10(6):606–14.
Carlin BI, Andriole GL. The natural history, skeletal complications, and management of bone metastases in patients with prostate carcinoma. Cancer. 2000;88(12 Suppl):2989–94.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47.
Lecouvet FE, Larbi A, Pasoglou V, et al. MRI for response assessment in metastatic bone disease. Eur Rad. 2013;23:1986–97.
Messiou C, Collins DJ, Giles S, De Bono JS. Bianchini, de Souza NM. Assessing response in bone metastases in prostate cancer with diffusion weighted MRI. Eur Rad. 2011;21:2169–77.
Messiou C, de Souza NM. Diffusion weighted magnetic resonance imaging of metastatic bone disease: a biomarker for treatment response monitoring. Cancer Biomarkers. 2010;6:21–32.
Messiou C, Collins DJ, Morgan VA, de Souza NM. Optimising diffusion weighted MRI for imaging metastatic and myeloma bone disease and assessing reproducibility. Eur Rad. 2011;21(8):1713–8.
Pollen JJ, Witztum KF, Ashburn WL. The flare phenomenon on radionuclide bone scan in metastatic prostate cancer. AJR. 1984;142:773–6.
Messiou C, Cook G, Reid AH, Attard G, Dearnaley D, de Bono JS, et al. The CT flare response of metastatic bone disease in prostate cancer. Acta Radiol. 2011;52(5):557–61.
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17(11):3461–7.
Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current induced distortion in diffusion MRI using twice refocused spin echo. Magn Reson Med. 2003;49(1):177–82.
Padhani AR, Liu G, Mu-Koh D, et al. Diffusion weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102–25.
Mundinger A, Wiesmeier B, Dinkel E, Helwig A, Beck A, Schulte Moenting J. Quantitative image analysis of vertebral body architecture-improved diagnosis in osteoporosis based on high-resolution computed tomography. Br J Radiol. 1993;66(783):209–13.
Park SH, Kim SJ, Park BC, Suh KJ, Lee JY, Park CW, et al. Three-dimensional osseous micro-architecture of the distal humerus: implications for internal fixation of osteoporotic fracture. J Should Elb Surg. 2010;19(2):244–50.
Acknowledgments
We acknowledge the support received for the CRUK and EPSRC Cancer Imaging Centre in association with the MRC and department of Health (England) grant C1060/A10334 and also NHS funding to the NIHR Biomedical Research Centre.
Conflict of interest
The authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Messiou, C., Collins, D.J., Morgan, V.A. et al. Use of apparent diffusion coefficient as a response biomarker in bone: effect of developing sclerosis on quantified values. Skeletal Radiol 43, 205–208 (2014). https://doi.org/10.1007/s00256-013-1768-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-013-1768-3