Abstract
Objective
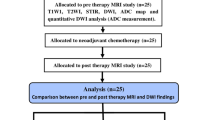
The objective of this study was to evaluate whether the average apparent diffusion coefficient (ADC) or the minimum ADC is more useful for evaluating the chemotherapeutic response of osteosarcoma.
Materials and methods
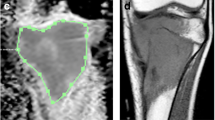
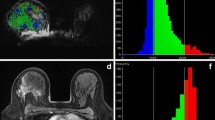
Twenty-two patients with osteosarcoma were examined in this study. Diffusion-weighted (DW) and magnetic resonance (MR) images were performed for all patients before and after chemotherapy. The pre- and post-chemotherapy values were obtained both in the average and minimum ADC. The pre-chemotherapy values of the average ADC and minimum ADC respectively were compared with the post-chemotherapy values. In addition, the ADC ratios ([ADCpost - ADCpre] / ADCpre) were calculated using the average ADC and the minimum ADC. Twenty-two patients with osteosarcomas were divided into two groups, those with a good response to chemotherapy (≥ 90% tumor necrosis, n = 7) and those with a poor response (< 90% tumor necrosis, n = 15). The average ADC ratio and the minimum ADC ratio of the two groups were compared.
Results
With both the average ADC and the minimum ADC, post-chemotherapy values were significantly higher than pre-chemotherapy values (P < 0.05). The patients with a good response had a significantly higher minimum ADC ratio than those with a poor response (1.01 ± 0.22 and 0.55 ± 0.29 respectively, P < 0.05). However, with regard to the average ADC ratio, no significant difference was observed between the two groups (0.66 ± 0.18 and 0.46 ± 0.31 respectively, P = 0.19).
Conclusion
The minimum ADC is useful for evaluating the chemotherapeutic response of osteosarcoma.



Similar content being viewed by others
References
Shen SH, Chiou YY, Wang JH, et al. Diffusion-weighted single-shot echo-planar imaging with parallel technique in assessment of endometrial cancer. AJR Am J Roentgenol. 2008;190:481–8.
Gourtsoyianni S, Papanikolaou N, Yarmenitis S, Maris T, Karantanas A, Gourtsoyiannis N. Respiratory gated diffusion-weighted imaging of the liver: value of apparent diffusion coefficient measurements in the differentiation between most commonly encountered benign and malignant focal liver lesions. Eur Radiol. 2008;18:486–92.
Manenti G, Di Roma M, Mancino S, et al. Malignant renal neoplasms: correlation between ADC values and cellularity in diffusion weighted magnetic resonance imaging at 3 T. Radiol Med. 2008;113:199–213.
Hayashida Y, Yakushiji T, Awai K, et al. Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol. 2006;16:2637–43.
Uhl M, Saueressig U, van Buiren M, et al. Osteosarcoma: preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol. 2006;41:618–23.
Murakami R, Sugahara T, Nakamura H, et al. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology. 2007;243:493–9.
Harris AD, Govindaraj M, Frayne R. Minimum detectable difference of MR diffusion maps in acute ischemic stroke. J Magn Reson Imaging. 2008;27:629–33.
Sugahara T, Korogi Y, Kochi M, et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging. 1999;9:53–60.
Provenzale JM, Mukundan S, Barboriak DP. Diffusion-weighted and perfusion MR imaging for brain tumor characterization and assessment of treatment response. Radiology. 2006;239:632–49.
Picci P, Bacci G, Campanacci M, et al. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer. 1985;56:1515–21.
De Baere T, Vanel D, Shapeero LG, Charpentier A, Terrier P, di Paola M. Osteosarcoma after chemotherapy: evaluation with contrast material-enhanced subtraction MR imaging. Radiology. 1992;185:587–92.
Huvos AG, Rosen G, Marcove RC. Primary osteogenic sarcoma: pathologic aspects in 20 patients after treatment with chemotherapy en bloc resection, and prosthetic bone replacement. Arch Pathol Lab Med. 1977;101:14–8.
Bacci G, Longhi A, Versari M, Mercuri M, Briccoli A, Picci P. Prognostic factors for osteosarcoma of the extremity treated with neoadjuvant chemotherapy: 15-year experience in 789 patients treated at a single institution. Cancer. 2006;106:1154–61.
Rosen G, Marcove RC, Huvos AG, et al. Primary osteogenic sarcoma: eight-year experience with adjuvant chemotherapy. J Cancer Res Clin Oncol. 1983;106:55–67.
Holscher HC, Hermans J, Nooy MA, Taminiau AH, Hogendoorn PC, Bloem JL. Can conventional radiographs be used to monitor the effect of neoadjuvant chemotherapy in patients with osteogenic sarcoma? Skeletal Radiol. 1996;25:19–24.
Kumpan W, Lechner G, Wittich GR, et al. The angiographic response of osteosarcoma following pre-operative chemotherapy. Skeletal Radiol. 1986;15:96–102.
Holscher HC, Bloem JL, Vanel D, et al. Osteosarcoma: chemotherapy-induced changes at MR imaging. Radiology. 1992;182:839–44.
Chenevert TL, Stegman LD, Taylor JM, et al. Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst. 2000;92:2029–36.
Mardor Y, Roth Y, Lidar Z, et al. Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res. 2001;61:4971–3.
Mardor Y, Pfeffer R, Spiegelmann R, et al. Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol. 2003;21:1094–100.
Deng J, Miller FH, Rhee TK, et al. Diffusion-weighted MR imaging for determination of hepatocellular carcinoma response to yttrium-90 radioembolization. J Vasc Interv Radiol. 2006;17:1195–200.
Theilmann RJ, Borders R, Trouard TP, et al. Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia. 2004;6:831–7.
National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas: summary and description of a working formulation for clinical usage—the non-Hodgkin’s lymphoma pathologic classification project. Cancer 1982; 49:2112–35.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oka, K., Yakushiji, T., Sato, H. et al. The value of diffusion-weighted imaging for monitoring the chemotherapeutic response of osteosarcoma: a comparison between average apparent diffusion coefficient and minimum apparent diffusion coefficient. Skeletal Radiol 39, 141–146 (2010). https://doi.org/10.1007/s00256-009-0830-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-009-0830-7