Abstract
Background
Histological necrosis, the current standard for response evaluation in osteosarcoma, is attainable after neoadjuvant chemotherapy.
Objective
To establish the role of surrogate markers of response prediction and evaluation using MRI in the early phases of the disease.
Materials and methods
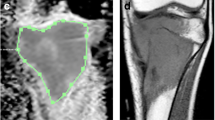
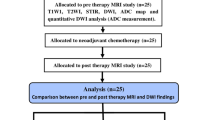
Thirty-one treatment-naïve osteosarcoma patients received three cycles of neoadjuvant chemotherapy followed by surgery during 2006–2008. All patients underwent baseline and post-chemotherapy conventional, diffusion-weighted and dynamic contrast-enhanced MRI. Taking histological response (good response ≥90% necrosis) as the reference standard, various parameters of MRI were compared to it. A tumor was considered ellipsoidal; volume, average tumor plane and its relative value (average tumor plane relative/body surface area) was calculated using the standard formula for ellipse. Receiver operating characteristic curves were generated to assess best threshold and predictability. After deriving thresholds for each parameter in univariable analysis, multivariable analysis was carried out.
Results
Both pre-and post-chemotherapy absolute and relative-size parameters correlated well with necrosis. Apparent diffusion coefficient did not correlate with necrosis; however, on adjusting for volume, significant correlation was found. Thus, we could derive a new parameter: diffusion per unit volume.
Conclusion
In osteosarcoma, chemotherapy response can be predicted and evaluated by conventional and diffusion-weighted MRI early in the disease course and it correlates well with necrosis. Further, newly derived parameter diffusion per unit volume appears to be a sensitive substitute for response evaluation in osteosarcoma.





Similar content being viewed by others
References
Saeter G, Hoie J, Stenwig AE et al (1995) Systemic relapse of patients with osteogenic sarcoma: prognostic factors for long term survival. Cancer 75:1084–1093
Enneking WF, Spanier SS, Goodman MA (1980) A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop 153:106–120
Miller AB, Hoogstraten B, Staquet M et al (1981) Reporting results of cancer treatment. Cancer 47:207–214
Uhl M, Saueressig U, Koehler G et al (2006) Evaluation of tumor necrosis during chemotherapy with diffusion-weighted MR imaging: preliminary results in osteosarcomas. Pediatr Radiol 36:1306–1311
Dudeck O, Zeile M, Pink D et al (2003) Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J Magn Reson Imaging 27:1109–1113
Moffat BA, Chenevert TL, Meyer CR et al (2006) The functional diffusion map: an imaging biomarker for the early prediction of cancer treatment outcome. Neoplasia 8:259–267
Koh DM, Collins DJ (2007) Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR 188:1622–1635
Schnall MD, Blume J, Bluemke DA et al (2006) Diagnostic architectural and dynamic features at breast MR imaging: multicenter study 1. Radiology 238:42–53
Greene FL, Page DL, Fleming ID et al (2002) AJCC cancer staging manual, 6th edn. Springer, New York
Picci P, Bacci G, Campanacci M et al (1985) Histological evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer 56:1515
Bacci G, Picci P, Ruggieri P et al (1990) Primary chemotherapy and delayed surgery (neoadjuvant chemotherapy) for osteosarcoma of the extremities. The Instituto Rizzoli experience in 127 patients treated preoperatively with intravenous methotrexate (high versus moderate doses) and intraarterial cisplatin. Cancer 65:2539–2553
Winkler K, Beron G, Delling G et al (1988) Neoadjuvant chemotherapy of osteosarcoma: results of a randomized cooperative trial (COSS-82) with salvage chemotherapy based on histological tumor response. J Clin Oncol 6:329–337
Withers HR, Lee SP (2006) Modeling growth kinetics and statistical distribution of oligometastases. Semin Radiat Oncol 16:111–119
Pervaiz S (2002) Anti-cancer drugs of today and tomorrow: are we close to making the turn from treating to curing cancer? Curr Pharm Des 8:1723–1734
Nathan SS, DiResta GR, Casas-Ganem JE et al (2005) Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res 11:2389–2397
Brisse H, Ollivier L, Edeline V et al (2004) Imaging of malignant tumors of the long bones in children: monitoring response to neoadjuvant chemotherapy and preoperative assessment. Pediatr Radiol 34:595–605
Jones DN, McCowage GB, Sostman HD et al (1996) Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med 37:1438–1444
Kim MS, Lee SY, Cho WH et al (2008) Initial tumor size predicts histologic response and survival in localized osteosarcoma patients. J Surg Oncol 97:456–461
Vander Woude HJ, Bloem JL, Hogendoorn PC (1998) Preoperative evaluation and monitoring chemotherapy in patients with high-grade osteogenic and Ewing’s sarcoma: review of current imaging modalities. Skeletal Radiol 27:57–71
Kim MS, Lee SY, Cho WH et al (2008) Tumor necrosis rate adjusted by tumor volume change is a better predictor of survival of localized osteosarcoma patients. Ann Surg Oncol 15:906–914
Lee JA, Kim MS, Kim DH et al (2008) Relative tumor burden predicts metastasis-free survival in pediatric osteosarcoma. Pediatr Blood Cancer 50:195–200
Groninger E, Proost JH, de Graaf SS (2004) Pharmacokinetic studies in children with cancer. Crit Rev Oncol Hematol 52:173–197
Kaste SC, Liu T, Billups CA et al (2004) Tumor size as a predictor of outcome in pediatric non-metastatic osteosarcoma of the extremity. Pediatr Blood Cancer 43:723–728
Uhl M, Saueressig U, van Buiren M et al (2006) Preliminary results of in vivo assessment of tumor necrosis after chemotherapy with diffusion- and perfusion-weighted magnetic resonance imaging. Invest Radiol 41:618–623
Hayashida Y, Yakushiji T, Awai K et al (2006) Monitoring therapeutic responses of primary bone tumors by diffusion-weighted image: initial results. Eur Radiol 16:2637–2643
Theilmann RJ, Borders R, Trouard TP et al (2004) Changes in water mobility measured by diffusion MRI predict response of metastatic breast cancer to chemotherapy. Neoplasia 6:831–837
Chenevert TL, Stegman LD, Taylor JM et al (2000) Diffusion magnetic resonance imaging: an early surrogate marker of therapeutic efficacy in brain tumors. J Natl Cancer Inst 92:2029–2036
Mardor Y, Pfeffer R, Spiegelmann R et al (2003) Early detection of response to radiation therapy in patients with brain malignancies using conventional and high b-value diffusion-weighted magnetic resonance imaging. J Clin Oncol 21:1094–1100
Deng J, Miller FH, Rhee TK et al (2006) Diffusion-weighted MR imaging for determination of hepatocellular carcinoma response to yttrium-90 radioembolization. J Vasc Interv Radiol 17:1195–1200
Dyke JP, Panicek DM, Healey JH et al (2003) Osteogenic and Ewing sarcomas: estimation of necrotic fraction during induction chemotherapy with dynamic contrast enhanced MR imaging. Radiology 228:271–278
Guo JY, Reddick WE (2009) DCE-MRI pixel-by-pixel quantitative curve pattern analysis and its application to osteosarcoma. J Magn Reson Imag 30:177–184
Shin KH, Moon SH, Suh JS et al (2000) Tumor volume change as a predictor of chemotherapeutic response in osteosarcoma. Clin Orthop Relat Res 376:200–208
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajpai, J., Gamnagatti, S., Kumar, R. et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol 41, 441–450 (2011). https://doi.org/10.1007/s00247-010-1876-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-010-1876-3