Abstract
Introduction
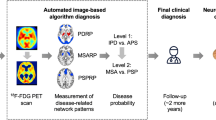
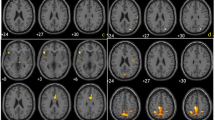
Positron emission tomography (PET) imaging with F-18 fluorodeoxyglucose (FDG) has been used to identify characteristic patterns of regional glucose metabolism in patients with idiopathic Parkinson's disease (IPD) and the atypical parkinsonian syndromes of progressive supranuclear palsy (PSP), multiple system atrophy (MSA), and corticobasal syndrome (CBS). We undertook this study to assess the utility of fluorodeoxyglucose-PET in the differential diagnosis of individual patients with clinical parkinsonism. “Visual” and “computer-supported” reading of the fluorodeoxyglucose-PET scans were used for image interpretation and compared with each other.
Methods
One hundred thirty-six parkinsonian patients were referred from movement disorder clinics in specialty neurology centers for the fluorodeoxyglucose-PET study. Imaging-based diagnosis was obtained by visual assessment of individual scans by a PET physician blinded to the clinical diagnosis and also by computer-assisted interpretation using statistical parametric mapping (SPM) analysis. The results were compared with a 2-year follow-up clinical assessment made by a movement disorder specialist.
Results
Concordance of visual evaluation of fluorodeoxyglucose-PET with clinical diagnosis was achieved in 91.7 % of patients scanned, 97.6 % IPD, 80 % MSA, 76.6 % PSP, and 100 % CBS. Blinded computer assessment using SPM was concordant with the clinical diagnosis in 91 % of cases evaluated (90.4 % IPD, 80 % MSA, 93.3 % PSP, and 100 % CBS).
Conclusions
Fluorodeoxyglucose-PET performed at the time of initial referral for parkinsonism is useful for the differential diagnosis of IPD, PSP, MSA, and CBS. Computer-assisted methods can be used for objective evaluation especially when expert readers are not available.



Similar content being viewed by others
References
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatr 55:181–184
Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of Parkinsonian syndromes in a specialist movement disorder service. Brain 125:861–870
Osaki Y, Wenning GK, Daniel SE, Hughes A, Lees AJ, Mathias CJ et al (2002) Do published criteria improve clinical diagnostic accuracy in multiple system atrophy? Neurology 59:1486–1491
Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T et al (1994) The metabolic topography of Parkinsonism. J Cereb Blood Flow Metab 14(5):783–801
Moeller JR, Nakamura T, Mentis MJ, Dhawan V, Spetsieris P, Antonini A et al (1999) Reproducibility of regional metabolic covariance patterns: comparison of four populations. J Nucl Med 40:1264–1269
Blin J, Baron JC, Dubois B, Pillon B, Cambon H, Cambiar J et al (1990) Positron emission tomography study in progressive supranuclear palsy. Brain hypometabolic pattern and clinicopathological correlations. Arch Neurol 47:747–752
Karbe H, Grond M, Huber M, Herholz K, Kessler J, Heiss WD (1992) Subcortical damage and cortical dysfunction in progressive supranuclear palsy demonstrated by positron emission tomography. J Neurol 239:98–102
Eidelberg D, Takikawa S, Moeller JR, Dhawan V, Redington K, Chaly T et al (1993) Striatal hypometabolism distinguishes striatonigral degeneration from Parkinson's disease. Ann Neurol 33:518–527
Antonini A, Kazumata K, Feigin A, Mandel F, Dhawan V, Margouleff C et al (1998) Differential diagnosis of Parkinsonism with F-18 fluorodeoxyglucose and PET. Mov Disord 13:268–274
Taniwaki T, Nakagawa M, Yamada T, Yoshida T, Ohyagi Y, Sasaki M et al (2002) Cerebral metabolic changes in early multiple system atrophy: a PET study. J Neurol Sci 200:79–84
Eidelberg D, Dhawan V, Moeller JR, Sidtis JJ, Ginos JZ, Strother SC et al (1991) The metabolic landscape of cortico-basal ganglionic degeneration: regional asymmetries studied with positron emission tomography. J Neurol Neurosurg Psychiatry 54:856–862
Laureys S, Salmon E, Garraux G, Peigneux P, Lemaire C, Degueldre C et al (1999) Fluorodopa uptake and glucose metabolism in early stages of corticobasal degeneration 246: 1151–1158
Dhawan V, Eidelberg D (2003) PET imaging in Parkinson's disease. In: Sinha KK, Chandra P, Jha DK (eds) Advances in clinical neurosciences. The Catholic Press, Jharkand, pp 251–276
Eckert T, Barnes A, Dhawan V, Frucht S, Gordon MF, Feigin AS et al (2005) FDG PET in the differential diagnosis of Pakinsonian disorders. NeuroImage 26:912–921
Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ (1992) What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study? Neurology 42:1142–1146
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL et al (1994) Preliminary NINDS neuropathologic criteria for Steele–Richardson–Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2018
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ et al (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
O’Sullivan SS, Massey LA, Williams DR, Silveria-Moriyama L, Kempster PA, Holton JL et al (2008) Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain 131:1362–1372
Shih LC, Tarsy D (2007) Deep brain stimulation for the treatment of atypical Parkinsonism. Mov Disord 22:2149–2155
Zhao P, Zhang B, Gao S (2012) F-18 FDG PET study on idiopathic Parkinson's disease from several parkinsonian plus syndromes. Parkinsonism Relat Disord 12:S60–S62
Burn DJ, Sawle GV, Brooks DJ (1994) Differential diagnosis of Parkinson's disease, multiple system atrophy, and Steele–Richardson–Olszewski syndrome: discriminant analysis of striatal F-18 FDOPA PET data. J Neurol Neurosurg Psychiatry 57:278–284
Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Chaly T et al (1995) Early differential diagnosis of Parkinson's disease with F-18 fluorodeoxyglucose and positron emission tomography. Neurology 45:1995–2004
Parent A, Hazrat LN (1995) Functional anatomy of the basal ganglia. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev 20:91–127
Alexander GE, Crutcher MD, DeLong MR (1990) Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. Progr Brain Res 85:119–146
Jellinger KA (1991) Pathology of Parkinson's disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14:153–197
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol aging 24:197–211
Gai WP, Halliday GM, Blumberg PC, Geffen LB, Blessing WW (1991) Substance P-containing neurons in the mesopontine tegmentum are severely affected in Parkinson's disease. Brain 114:2253–2267
Chinaglia G, Landwehrmeyer B, Probst A, Palacios JM (1993) Serotoninergic terminal transporters are differently affected in Parkinson's disease and progressive supranuclear palsy: an autographic study with [H-3] citalopram. Neuroscience 54(3):691–699
Acknowledgments
This work was funded by a fellowship grant to Dr. Madhavi Tripathi from the Indo-US Science and Technology Form. We are grateful to Dr. David Eidelberg and Dr. Yilong Ma of the Functional Imaging Laboratory, The Feinstein Institute for Medical Research, NSLIJHS, New York, for their critical review and expert guidance. We wish to acknowledge Dr. RP Tripathi, Director INMAS, and the entire team of Division of PET Imaging, INMAS for feasibility of patient studies. The authors also wish to thank Dr. Deepak Gupta for his initial contribution to this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tripathi, M., Dhawan, V., Peng, S. et al. Differential diagnosis of parkinsonian syndromes using F-18 fluorodeoxyglucose positron emission tomography. Neuroradiology 55, 483–492 (2013). https://doi.org/10.1007/s00234-012-1132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-012-1132-7