Abstract
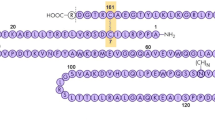
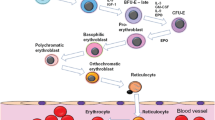
Cloning of the human erythropoietin (EPO) gene and development of the first recombinant human erythropoietin (rHuEPO) drug were truly breakthroughs. This allowed a deeper understanding of the structure and pharmacology of rHuEpo, which in turn inspired the discovery and development of additional erythropoiesis-stimulating agents (ESAs). In vivo specific activity and serum half-life of rHuEPO are influenced by the amount and structure of the attached carbohydrate. Increased numbers of sialic acids on carbohydrate attached to rHuEPO correlated with a relative increase in in-vivo-specific activity and increased serum half-life. The effect of increasing the number of sialic-acid-containing carbohydrates on in-vivo-specific activity was explored. Initial research focused on solving the problem of how the protein backbone could be engineered so a cell would add more carbohydrate to it. Additional work resulted in darbepoetin alfa, a longer-acting molecule with two additional carbohydrate chains.







Similar content being viewed by others
References
Hebbel RP, Eaton JW (1989) Pathobiology of heme interaction with the erythrocyte membrane. Semin Hematol 26:136–149
Egrie JC, Browne JK (2001) Development and characterization of novel erythropoiesis stimulating protein (NESP). Nephrol Dial Transplant 16(Suppl 3):3–13
Elliott S, Heatherington AC, Foote M (2004) Cancer drug discovery and development hematopoietic growth factors in oncology: basic science and clinical therapeutics, 6 chapter, Erythropoietic factors-Clinical pharmacology and pharmacokinetics. Human Press Inc, Totowa, NJ, pp 97–123
Erslev A (1952) Humoral regulation of red cell production. Blood 8:349–357
Miyake T, King CK, Goldwasser E (1977) Purification of human erythropoietin. J Biol Chem 252:5558–5564
Lin FK, Suggs S, Lin CH, Browne JK, Smalling R, Egrie JC (1985) Cloning and expression of the human erythropoietin gene. Proc Natl Acad Sci USA 82:7580–7584
Lai PH, Everett R, Wang FF, Arakawa T, Goldwasser E (1986) Structural characterization of human erythropoietin. J Biol Chem 261:3116–3121
Syed RS, Reid SW, Li C et al (1998) Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature 395:511–516
Jiang BH, Rue E, Wang GL, Roe R, Semenza GL (1996) Dimerization, DNA binding and transactivation properties of hypoxia-inducible factor 1. J Biol Chem 271:17771–17778
Davis JM, Arakawa T, Strickland TW, Yphantis DA (1987) Characterization of recombinant human erythropoietin produced in Chinese hamster ovary cells. Biochemistry 26:2633–2638
Elliott S, Lorenzini T, Chang D, Barzilay J, Delorme E (1997) Mapping of active site of recombinant human erythropoietin. Blood 89:493–502
Watowich SS (1999) Activation of erythropoietin signaling by receptor dimerization. Int J Biochem Cell Biol 31:1075–1088
Alfrey CP, Fishbane S (2007) Implications of neocytolysis for optimal management of anaemia in chronic kidney disease. Nephron Clin Pract 106:c149–c156
Sinclair A, Coxon A, McCaffery I et al (2010) Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal and renal cells. Blood, In press
Landaw SA, Leon HA, Winchell HS (1970) Effects of hyperoxia on red blood cell survival in the normal rat. Aerosp Med 41(1):48–55
Fisher CL, Johson PC, Berry CA (1967) Red blood cell mass and plasma volume changes in manned space flight. JAMA 200(7):579–583
Berry CA (1969) Preliminary clinical report of the medical aspects of Apollos VII and VIII. Aeros Med 40:245–254
Hattangadi SM, Lodish HF (2007) Regulation of erythrocyte lifespan: do reactive oxygen species set the clock? J Clin Invest 117:2075–2077
Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S (2007) Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest 117:2133–2144
Wu H, Lee SH, Gao J, Liu X, Iruela-Arispe ML (1999) Inactivation of erythropoietin leads to defects in cardiac morphogenesis. Development 126(16):3597–3605
Erbayraktar S, Yilmaz O, Gokmen N, Brines M (2003) Erythropoietin is a multifunctional tissue-protective cytokine. Current Hematology Reports 2(6):465–470
Logar CM, Brinkkoetter PT, Krofft RD, Pippin JW, Shankland SJ (2007) Darbepoetin alfa protects podocytes from apoptosis in vitro and in vivo. Kidney Int 72(4):489–498
Eto N, Wada T, Inagi R, Takano H, Shimizu A, Kato H, Kurihara H, Kawachi H, Shankland SJ, Fujita T, Nangaku M (2007) Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int 72(4):455–463
Jelkmann W, Bohlius J, Hallek M, Sytkowskí AJ (2008) The erythropoietin receptor in normal and cancer tissue. Crit Rev Oncol Hematol 67:39–61
Sinclair AM, Todd MD, Forsythe K et al (2007) Expression and function of erythropoietin receptors in tumors: implications for the use of erythropoiesis-stimulating agents in cancer patients. Cancer 110:477–488
Glaspy JA (2008) Erythropoiesis-stimulating agents in oncology. J Natl Compr Canc Netw 6:565–575
Brown WM, Maxwell P, Graham AN, Yakkundi A, Dunlop EA, Shi Z, Johnston PG, Lappin TR (2007) Erythropoietin receptor expression in non-small cell lung carcinoma: a question of antibody specificity. Stem Cells 25(3):718–722
Elliott S, Busse L, Bass MB, Lu H et al (2006) Anti-Epo receptor antibodies do not predict Epo receptor expression. Blood 107:1892–1895
Suzuki N, Ohneda O, Takahashi S, Higuchi M, Mukai HY, Nakahata T, Imagawa S, Yamamoto M (2002) Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood 100(7):2279–2288
Laugsch M, Metzen E, Svensson T, Depping R, Jelkmann W (2008) Lack of functional erythropoietin receptors of cancer cell lines. Int J Cancer 122:1005–1011
Elliott S, Busse L, McCaffery I et al (2009) Identification of sensitive anti-erythropoietin receptor monoclonal antibody allows detection of low levels of EpoR in cells. J Immunol Methods. doi: 10.1016/j.jim.2009.10.006
Swift S, Elliott S, Sinclair A, Begley CG (2009) Erythropoietin receptor in ovarian cancer cells. Mol Cancer Ther
Rossi J, McCaffery I, Paweletz K et al (2009) Analysis of cell surface erythropoietin receptor (EpoR) expression and function in human epithelial tumor tissues. J Clin Oncol 27:abstr 11104
Katavetin P, Tungsanga K, Eiam-Ong S, Nangaku M (2007) Antioxidative effects of erythropoietin. Kidney Int Suppl 107:S10–S15
Schellekens Huub (2009) Biosimilar therapeutics-what do we need to consider? Nephrol Dial Transplant Plus 2(Suppl 1):i27–i36
Schellekens H (2005) Follow-on biologics: Challenges of the 'next generation'. Nephrol Dial Transplant 20:iv31–iv36
Jelkmann W (2009) Efficacy of recombinant erythropoietins: is there unity of international units? Nephrol Dial Transplant 24:1366–1368
Park SS, Park J, Ko J et al (2009) Biochemical assessment of erythropoietin products from Asia versus US Epoetin alfa manufactured by Amgen. J Pharm Sci 98:1688–1699
Elliott S, Egrie J, Browne J, Lorenzini T, Busse L, Rogers N, Ponting I (2004) Control of rHuEPO biological activity: The role of carbohydrate. Exp Hematol 32:1146–1155
Strickland T, Adler B, Aoki K, Asher S, Derby P, Goldwasser E, Rogers G (1992) Occurrence of sulfate on the N-linked oligosaccharides of human erythropoietin. J Cell Biochem Supplement 16D:167, Ref Type: Abstract
Lasne F, Martin L et al (2002) Detection of isoelectric profiles of erythropoietin in urine: Differentiation of natural and administered recombinant hormones. Anal Biochem 311:119–12
Egrie JC, Browne JK (2001) Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer 84(Suppl 1):3–10
Elliott S, Chang D, Delorme E, Eris T, Lorenzini T (2004) Structural requirements for additional N-linked carbohydrate on recombinant human erythropoietin. J Biol Chem 279:16854–16862
Delorme E, Lorenzini T, Giffin J et al (1992) Role of glycosylation on the secretion and biological activity of erythropoietin. Biochemistry 31:9871–9876
Elliott S, Lorenzini T, Asher S, Aoki K, Brankow D, Buck L, Busse L et al (2003) Enhancement of therapeutic protein in vivo activities glycoengineering. Nature 21:414–421
Agoram B, Aoki K, Doshi S, Gegg Co, Jang G, Molineux G, Narhi L, Elliott S (2009) Investigation of the Effects of Altered Receptor Binding Activity on the Clearance of Erythropoiesis-Stimulating Proteins: Nonerythropoietin Receptor-Mediated Pathways May Play a Major Role. J Pharm Sci 98(6):2198–2211
Egrie JC, Dwyer E, Browne JK, Hitz A, Lykos MA (2003) Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp Hematol 31:290–299
Macdougall IC, Gray SJ, Elston O et al (1999) Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol 10:2392–2395
Glaspy J, Henry D, Patel R et al (2005) Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: a randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. Eur J Cancer 41:1140–1149
Allon M, Kleinman K, Walczyk M, Kaupke C, Messer-Mann L, Olson K, Heatherington AC, Maroni BJ (2002) Pharmacokinetics and pharmacodynamics of darbepoetin alfa and epoetin in patients undergoing dialysis. Clin Pharmacol Ther 72:546–555
Macdoughall IC (2002) Optimizing the use of erythropoietic agents-pharmacokinetic and pharmacodynamic considerations. Nephrol Dial Transplant 17(Suppl 5):66–70
Mann J, Kessler M, Villa G (2007) Darbepoetin alfa once every 2 weeks for treatment of anemia in dialysis patients: a combined analysis of eight multicenter trials. Clin Nephrol 67:140–148
Sinclair AM, Elliott S (2005) Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci 94:1626–1635
Hartley C, Eliott S, Begley G, McElroy P, Sutherland W, Khaja R, Heatherington AC, Graves T, Schultz H, Castilio JD, Molineux G (2003) Kinetics of haematopoietic recovery after dose-intensive chemo/radiotherapy in mice: Optimized erythroid support with darbepoetin alpha. Br J Haematol 122:623–636
Sasu BJ, Hartley C, Schultz H et al (2005) Comparison of epoetin alfa and darbepoetin alfa biological activity under different administration schedules in normal mice. Acta Haematol 113:163–174
Tolman C, Richardson D, Bartlett C, Will E (2005) Structured conversion from thrice weekly to weekly erythropoietic regimens using a computerized decision-support system: a randomized clinical study. J Am Soc Nephrol 16:1463–1470
Carrera F, Oliveira L (2006) The efficacy of intravenous darbepoetin alfa administered once every 2 weeks in chronic kidney disease patients on hemodialysis. Nephrol Dial Transplant 21:2846–2850
Ling B, Walczyk M (2005) Darbepoetin alfa administered once monthly maintains haemoglobin concentration in patients with chronic kidney disease. Clin Nephrol 63:327–334
Gross AW, Lodish HF (2006) Cellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP). J Biol Chem 281:2024–2032
Eliott S, Pham E, Macdougall IC (2008) Erythropoiesis: a common mechanism of action. Exp Hematol 36:1573–1584
Jarsch M, Brandt M, Lanzendorfer M, Haselbeck A (2008) Comparative erythropoietin receptor binding kinetics of C.E.R.A. and epoetin-beta determined by surface plasmon resonance and competition binding assay. Pharmacology 81:63–69
Macdougall IC et al. (2006) Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol 1:1211–1215
Fan Q, Leuther KK et al (2006) Preclinical evaluation of Hematide, a novel erythropoiesis stimulating agent, for the treatment of anemia. Exp Hematol 34:1303–1311
Stead RB, Lambert J, Wessels D et al (2006) Evaluation of the safety and pharmacodynamics of Hematide, a novel erythropoietic agent, in a phase 1, double-blind, placebo-controlled, dose-escalation study in healthy volunteers. Blood 108:1830–1834
Acknowledgements
The manuscript was sponsored by Amgen (Hungary). Vladimir Tesar participated in the advisory board for Amgen and Novartis; received a research grant from Amgen and lecture fees from Amgen, Astra Zeneca, Fresenius Kabi, Novartis, Roche and Teva. János Szegedi received consulting fees from Amgen and Janssen Cilag and participated in the advisory board for Amgen. Zoltán Kiss, Steven Elliott, Kinga Jedynasty are employees of Amgen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kiss, Z., Elliott, S., Jedynasty, K. et al. Discovery and basic pharmacology of erythropoiesis-stimulating agents (ESAs), including the hyperglycosylated ESA, darbepoetin alfa: an update of the rationale and clinical impact. Eur J Clin Pharmacol 66, 331–340 (2010). https://doi.org/10.1007/s00228-009-0780-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-009-0780-y