Abstract
Summary
The aim of this study was to examine the effects of bisphosphonate discontinuation on bone metabolism at the spine and hip measured using 18 F-fluoride PET. Bone metabolism at the spine remained stable following discontinuation of alendronate and risedronate at 1 year but increased in the hip in the alendronate group only.
Introduction
Bisphosphonates such as alendronate (ALN) or risedronate (RIS) have persistent effects on spine BMD following discontinuation.
Methods
Positron emission tomography (PET) was used to examine regional bone metabolism in 20 postmenopausal women treated with ALN (n = 11) or RIS (n = 9) for a minimum of 3 years at screening (range 3–9 years, mean 5 years for both groups). Subjects underwent a dynamic scan of the lumbar spine and a static scan of both hips at baseline and 6 and 12 months following treatment discontinuation. 18 F-fluoride plasma clearance (Ki) at the spine was calculated using a three-compartment model. Standardised uptake values (SUV) were calculated for the spine, total hip, femoral neck and femoral shaft. Measurements of BMD and biochemical markers of bone turnover were also performed.
Results
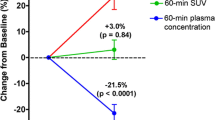
With the exception of a significant decrease in spine BMD in the ALN group, BMD remained stable. Bone turnover markers increased significantly from baseline by 12 months for both study groups. Measurements of Ki and SUV at the spine and femoral neck did not change significantly in either group. SUV at the femoral shaft and total hip increased significantly but in the ALN group only, increasing by 33.8% (p = 0.028) and 24.0% (p = 0.013), respectively.
Conclusions
Bone metabolism at the spine remained suppressed following treatment discontinuation. A significant increase in SUV at the femoral shaft and total hip after 12 months was observed but for the ALN group only. This study was small, and further clinical studies are required to fully evaluate the persistence of BP treatment.




Similar content being viewed by others
References
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR (2000) Fracture Intervention Trial. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FITResearch Group. J Clin Endocrinol Metab 85:4118–4124
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Alendronate Phase III Osteoporosis Treatment Study Group. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Chesnut CH III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, Felsenberg D, Huss H, Gilbride J, Schimmer RC, Delmas PD (2004) Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE). Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res 19:1241–1249
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY (2001) Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
Mellström DD, Sörensen OH, Goemaere S, Roux C, Johnson TD, Chines AA (2004) Seven years of treatment with risedronate in women with postmenopausal osteoporosis. Calcif Tissue Int 75:462–468
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Lawson MA, Xia Z, Barnett BL, Triffitt JT, Phipps RJ, Dunford JE, Locklin RM, Ebetino FH, Russell RG (2010) Differences between bisphosphonates in binding affinities for hydroxyapatite. J Biomed Mater Res B Appl Biomater 92:149–155
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Rizzoli R. Bisphosphonates for post-menopausal osteoporosis: are they all the same? QJM. 2011. Published online January 21 2011. [Epub ahead of print]
Russell RG (2006) Bisphosphonates: from bench to bedside. Ann N Y Acad Sci 1068:367–401
Russell RG, Watts NB, Ebetino FH, Rogers MJ (2008) Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int 19:733–759
Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SM, Sebba A, Kagan R, Chen E, Thompson DE, de Papp AE (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2637
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE (2005) Fosamax Actonel Comparison Trial Investigators. Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R (2007) Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int 18:25–34
Black DM, Boonen S, Delmas P, Lyles KW (2008) Review of comparative effectiveness of treatments to prevent fractures. Ann Intern Med 148:885–886
Rodan G, Reszka A, Golub E, Rizzoli R (2004) Bone safety of long-term bisphosphonate treatment. Curr Med Res Opin 20:1291–1300
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR (2006) FLEX Research Group. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Ensrud KE, Barrett-Connor EL, Schwartz A, Santora AC, Bauer DC, Suryawanshi S, Feldstein A, Haskell WL, Hochberg MC, Torner JC, Lombardi A, Black DM (2004) Fracture Intervention Trial Long-Term Extension Research Group. Randomized trial of effect of alendronate continuation versus discontinuation in women with low BMD: results from the Fracture Intervention Trial long-term extension. J Bone Miner Res 19:1259–1269
Greenspan SL, Emkey RD, Bone HG, Weiss SR, Bell NH, Downs RW, McKeever C, Miller SS, Davidson M, Bolognese MA, Mulloy AL, Heyden N, Wu M, Kaur A, Lombardi A (2002) Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 137:875–883
Ravn P, Bidstrup M, Wasnich RD, Davis JW, McClung MR, Balske A, Coupland C, Sahota O, Kaur A, Daley M, Cizza G (1999) Alendronate and estrogen-progestin in the long-term prevention of bone loss: four-year results from the early postmenopausal intervention cohort study. A randomized, controlled trial. Ann Intern Med 131:935–942
Stock JL, Bell NH, Chesnut CH 3rd, Ensrud KE, Genant HK, Harris ST, McClung MR, Singer FR, Yood RA, Pryor-Tillotson S, Wei L, Santora AC 2nd (1997) Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med 103:291–297
Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD (2000) Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab 85:3109–3115
Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC Jr (1998) Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab 83:396–402
Watts NB, Chines A, Olszynski WP, McKeever CD, McClung MR, Zhou X, Grauer A (2008) Fracture risk remains reduced one year after discontinuation of risedronate. Osteoporos Int 19:365–372
Cook GJR, Blake GM, Marsden PK, Cronin B, Fogelman I (2002) Quantification of skeletal kinetic indices in Paget’s disease using dynamic 18F-fluoride positron emission tomography. J Bone Miner Res 17:854–859
Frost ML, Cook GJ, Blake GM, Marsden PK, Benatar NA, Fogelman I (2003) A prospective study of risedronate on regional bone metabolism and blood flow at the lumbar spine measured by 18F-fluoride positron emission tomography. J Bone Miner Res 18:2215–2222
Frost ML, Fogelman I, Blake GM, Marsden PK, Fogelman I (2004) Dissociation between global markers of bone formation and direct measurement of spinal bone formation in osteoporosis. J Bone Miner Res 19:1797–1804
Frost ML, Cook GJR, Blake GM, Marsden PK, Fogelman I (2006) The relationship between regional bone turnover measured using 18F-fluoride positron emission tomography and changes in BMD is equivalent to that seen for biochemical markers of bone turnover. J Clin Densitom 10:46–54
Frost ML, Blake GM, Cook GJ, Marsden PK, Fogelman I (2009) Differences in regional bone perfusion and turnover between lumbar spine and distal humerus: (18)F-fluoride PET study of treatment-naïve and treated postmenopausal women. Bone 45:942–948
Frost ML, Siddique M, Blake GM, Moore AE, Schleyer PJ, Dunn JT, Somer EJ, Marsden PK, Eastell R, Fogelman I (2011) Differential effects of teriparatide on regional bone formation using 18F-fluoride positron emission tomography. J Bone Miner Res 26:1002–1011
Installe J, Nzeusseu A, Bol A, Depresseux G, Devogelaer JP, Lonneux M (2005) 18F-fluoride PET for monitoring therapeutic response in Paget's disease of bone. J Nucl Med 46:1650–1658
Messa C, Goodman WG, Hoh CK, Choi Y, Nissenson AR, Salusky IB, Phelps ME, Hawkins RA (1993) Bone metabolic activity measured with positron emission tomography and 18F-fluoride ion in renal osteodystophy: correlation with bone histomorphometry. J Clin Endo Metab 77:949–955
Piert M, Zittel TT, Becker GA, Jahn M, Stahlschmidt A, Maier G, Machulla H-J, Bares R (2001) Assessment of porcine bone metabolism by dynamic 18F-fluoride PET: correlation with bone histomorphometry. J Nucl Med 42:1091–1100
Piert M, Zittel TT, Jahn M, Stahlschmidt A, Becker GA, Machulla H-J (2003) Increased sensitivity in detection of a porcine high-turnover osteopenia after total gastrectomy by dynamic 18F-fluoride ion PET and quantitative CT. J Nucl Med 44:117–124
Schiepers C, Nuyts J, Bormans G, Dequeker J, Bouillon R, Mortelmans L, Verbruggen A, de Roo M (1997) Fluoride kinetics of the axial skeleton measured in vivo with fluorine-18-fluoride PET. J Nucl Med 38:1970–1976
Uchida K, Nakajima H, Miyazaki T et al (2009) Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by 18F-fluoride PET: a prospective study. J Nucl Med 50:1808–1814
Patel R, Blake GM, Rymer J et al (2000) Long-term precision of DXA scanning assessed over seven years in forty postmenopausal women. Osteoporos Int 11:68–75
Looker AC, Johnston CC Jr, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Lindsay RL (1995) Prevalence of low femoral bone density in older U.S. women from NHANES III. J Bone Miner Res 10:796–802
Cook GJR, Lodge MA, Marsden PK, Dynes A, Fogelman I (1999) Non-invasive assessment of skeletal kinetics using fluorine-18-fluoride positron emission tomography: evaluation of image and population-derived arterial input functions. Eur J Nuc Med 26:1424–1429
Hawkins RA, Choi Y, Huang S-C et al (1992) Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med 33:633–642
Frost ML, Blake GM, Park-Holohan SJ, Cook GJ, Curran KM, Marsden PK, Fogelman I (2008) Long-term precision of 18F-fluoride PET skeletal kinetic studies in the assessment of bone metabolism. J Nucl Med 49:700–707
Glover SJ, Eastell R, McCloskey EV et al (2009) Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone 45:1053–1058
Glover SJ, Garnero P, Naylor K, Rogers A, Eastell R (2008) Establishing a reference range for bone turnover markers in young, healthy women. Bone 42:623–630
Briot K, Trémollières F, Thomas T, Roux C (2007) Comité scientifique du GRIO. How long should patients take medications for postmenopausal osteoporosis? Joint Bone Spine 74:24–31
McClung MR (2007) Bisphosphonate therapy: to stop or not to stop? BoneKEy-Osteovision 4:78–82
Schmidt GA, Horner KE, McDanel DL, Ross MB, Moores KG (2010) Risks and benefits of long-term bisphosphonate therapy. Am J Health Syst Pharm 67:994–1001
Sebba A (2008) Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes 15:502–507
Fuchs RK, Phipps RJ, Burr DB (2008) Recovery of trabecular and cortical bone turnover after discontinuation of risedronate and alendronate therapy in ovariectomized rats. J Bone Miner Res 23:1689–1697
Wronski TJ, Dann LM, Qi H, Yen CF (1993) Skeletal effects of withdrawal of estrogen and diphosphonate treatment in ovariectomized rats. Calcif Tissue Int 53:210–216
Acknowledgements
We would like to thank the staff at the King’s College London PET Imaging Centre at St Thomas’ Hospital for their excellent technical support. This work was supported by an unrestricted grant from Warner Chilcott.
Conflicts of interest
Drs. Frost, Blake and Fogelman received research funding from Warner Chilcott to complete this study. Dr. Frost has received research funding from Eli Lilly, Warner Chilcott and Novartis. Dr. Fogelman has received research funding from Eli Lilly, Novartis, NPS, Procter & Gamble and Warner Chilcott. Dr. Eastell received consulting fees from Amgen, Novartis, Pfizer, Procter & Gamble, Servier, Ono and GSK; lecture fees from Eli Lilly and grant support from AstraZeneca, Procter & Gamble, Warner Chilcott, Amgen and Novartis. All other authors have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frost, M.L., Siddique, M., Blake, G.M. et al. Regional bone metabolism at the lumbar spine and hip following discontinuation of alendronate and risedronate treatment in postmenopausal women. Osteoporos Int 23, 2107–2116 (2012). https://doi.org/10.1007/s00198-011-1805-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-011-1805-9