Abstract
Aims/hypothesis
South Asians have a disproportionately high risk of developing abdominal obesity, insulin resistance and type 2 diabetes. Brown adipose tissue (BAT) has been identified as a possible target to fight obesity and protect against metabolic disturbance. We explored whether lower BAT activity in South Asians compared with Europids may contribute to the high risk of metabolic disturbance.
Methods
We studied 20 healthy men (ten Europids/ten South Asians, BMI 19–25 kg/m2, age 18–32 years). Following 2 h of cold exposure (16–18°C) after an overnight fast, 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography-computed tomography (CT) and 123I-metaiodobenzylguanidine (123I-MIBG) single-photon emission computed tomography-CT were performed to visualise metabolic BAT activity and sympathetic stimulation of BAT. Metabolic BAT activity was defined as maximal standardised uptake value (SUVmax) of 18F-FDG, and sympathetic stimulation of BAT as semiquantitative uptake value (SQUV) of 123I-MIBG. We performed hyperinsulinaemic–euglycaemic clamps to assess insulin sensitivity. Spearman’s correlations for SUVmax of 18F-FDG and both SQUV of 123I-MIBG and insulin sensitivity were determined.
Results
The median (interquartile range) SUVmax of 18F-FDG in South Asians (7.5 [2.2–10.6] g/ml) was not different from the median SUVmax obtained in Europids (4.5 [2.2–8.4] g/ml; p = 0.59). There was no correlation between BAT activity and insulin sensitivity. Correlations between SQUV of 123I-MIBG and SUVmax of 18F-FDG were positive, both in the total population (ρ = 0.80, p < 0.001) and after stratification by ethnicity (Europids, ρ = 0.65, p = 0.04; South Asians, ρ = 0.83, p = 0.01).
Conclusions/interpretation
This is the first study to prospectively investigate ethnic differences in metabolic BAT activity during cold exposure. We did not find differences in BAT activity between South Asians and Europids. Therefore, it seems unlikely that BAT plays an important role in the development of unfavourable metabolic profiles in South Asians.
Similar content being viewed by others
Introduction
Several studies have shown that South Asians have a disproportionately high risk of developing abdominal obesity, insulin resistance and type 2 diabetes [1–4]. Next to the higher prevalence, these metabolic disturbances also seem to develop at an earlier age in South Asians than in populations of European origin. The explanation for these ethnic differences is complex and only partially clear [3, 5].
Given its high capacity to dissipate excess energy, brown adipose tissue (BAT) has recently been identified as a possible target to fight obesity and protect against metabolic disturbance [6–9]. As such, ethnic differences in BAT activity might be a contributing factor to the adverse metabolic profile in South-Asian people. So far, data on ethnic differences in the activity of BAT in humans are limited to one retrospective study that found no significant difference in the prevalence of BAT between white and black patients with cancer [10]. However, only a limited number of black patients were included in this study. Furthermore, as BAT is optimally visualised during cold exposure and the patients included in this retrospective study were not exposed to cold, only BAT detected incidentally was assessed [10]. Therefore, BAT, while present, may not have been detected in some of the patients, thereby masking true ethnic differences in BAT activity.
We aimed to explore whether a lower BAT activity in South Asians, in comparison with Europids, may contribute to this high risk of metabolic disturbance. In addition, we compared the insulin sensitivity and sympathetic stimulation of BAT, the latter being an important determinant of BAT activity, between South Asians and Europids.
Methods
We studied a group of 20 (ten Europid, ten South Asian) healthy, lean, male volunteers (age 18–32 years, BMI 19–25 kg/m2). All the South-Asian men were Hindustani-Surinamese. The term ‘Hindustani-Surinamese’ refers to people of South-Asian ancestral origin and their offspring who migrated to the Netherlands via Surinam (a former colony of the Netherlands). The study participants were considered South Asian if both their parents, as well as their four grandparents, were of South-Asian origin. Nine out of the ten South-Asian participants were born in the Netherlands.
The study participants were recruited through public advertisements. All underwent a physical examination and a fasting blood sample was drawn. In order to determine the metabolic activity (i.e. glucose uptake) of BAT, the participants underwent 18F-fluorodeoxyglucose (18F-FDG) positron-emission tomography (PET)-computed tomography (CT). For the assessment of the sympathetic stimulation of BAT, a 123I-metaiodobenzylguanidine (123I-MIBG) single-photon emission computed tomography (SPECT)-CT was performed. As two South Asians did not complete their last study visit (for reasons unrelated to the study protocol), 123I-MIBG SPECT-CT was performed in 18 of the 20 study participants.
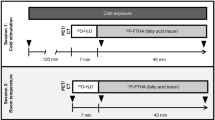
Both 18F-FDG and 123I-MIBG were administered after an overnight fast. The interval between 18F-FDG PET-CT and 123I-MIBG SPECT-CT was set between 1 and 2 weeks. To overcome any order bias 18F-FDG PET-CT and 123I-MIBG SPECT-CT were performed in random order. Furthermore, as healthy South Asians are less insulin sensitive than their European counterparts at a given BMI and age [11], and insulin sensitivity might influence BAT activity [12], we performed hyperinsulinaemic–euglycaemic clamps to assess insulin sensitivity in both ethnic groups. The institutional ethics committee of the Academic Medical Center approved the study protocol and all participants provided written informed consent.
Anthropometric and laboratory measurements
Weight was recorded in light clothing on a SECA mechanical scale to the nearest 100 g (SECA, Hamburg, Germany). Height was recorded to the nearest 0.01 m. Blood pressure was measured in seated position (Omron-M5-I, Omron Corporation, Kyoto, Japan). HbA1c was measured by ion-exchange chromatography on a Tosoh-G8 analyser (Tosoh Bioscience, Tokyo, Japan). Levels of fasting plasma glucose (FPG), insulin (sandwich enzyme immunoassay; Roche Diagnostics, Rotzkreuz, Switzerland), total cholesterol, HDL-cholesterol, LDL-cholesterol, triacylglycerol (enzymatic colorimetric method for all cholesterol and triacylglycerol measurements, Roche Diagnostics) and creatinine (colorimetric) were assessed.
18 F-FDG PET-CT scanning protocol
All participants were exposed to mild cold (∼17°C, controlled by use of an air conditioning system) for the duration of 2 h. Shivering was neither reported by participants nor noticed by research staff. After 1 h of cold exposure, approximately 200 MBq of 18F-FDG was administered intravenously and the cold exposure was continued for another hour. Upper-body (from base of the skull to groin) static PET was performed 60 min after 18F-FDG injection.
PET-CT images were acquired using a Gemini time-of-flight multidetector helical PET-CT scanner (2 min/bed position) (Philips Medical Systems, Eindhoven, the Netherlands). In areas where 18F-FDG uptake was identified by PET and the presence of fat was identified by CT (Hounsfield units between −250 and −50), the mean standardised uptake value (SUVmean) and the maximal standardised uptake value (SUVmax), defined as activity (Bq/ml) within the region of interest (ROI) ÷ injected dose (Bq/g body weight), were determined (Hybrid Viewer, Hermes Medical Solutions, Stockholm, Sweden). Anatomical ROIs were the cervical, supraclavicular and superior mediastinal depots. In these areas an SUV of 18F-FDG of at least 2.0 g/ml was considered to indicate BAT [6].
123 I-MIBG-SPECT-CT scanning protocol
All participants were pretreated with potassium iodide to block thyroid uptake of 123I-MIBG. Again, the participants were exposed to mild cold for 2 h. After 1 h of cold exposure, approximately 185 MBq of 123I-MIBG was administered intravenously; exposure to cold was continued for another hour. The next day, 24 h after 123I-MIBG injection, a SPECT-CT scan was performed at the same anatomical region [13].
An Infinia SPECT-CT imaging system (General Electric, Fairfield, CT, USA) with a medium-energy all-purpose collimator and a 128 × 128 matrix was used to acquire SPECT images. A 15% window was set for the main energy peak of 123I (159 keV). SPECT images were iteratively reconstructed (using ordered subset expectation maximisation) and corrected for attenuation using low-dose CT (no intravenous contrast). In areas where uptake of 123I-MIBG was identified by SPECT and the presence of fat was identified by CT, both the mean and semiquantitative uptake of 123I-MIBG were calculated as, respectively, the mean or maximal (decay corrected) count per voxel in the volume of interest (VOI) ÷ mean count per voxel in a reference region (i.e. the mediastinum) (Hybrid Viewer) [13].
Alignment of 18 F-FDG PET-CT and 123 I-MIBG SPECT-CT
The 18F-FDG PET-CT and 123I-MIBG SPECT-CT were aligned using the CT images with Hybrid Viewer. The results of this automated non-rigid registration algorithm were visually validated. The specific VOIs on the anatomical images of 18F-FDG PET-CT in which metabolically active BAT was present were copied to the aligned 123I-MIBG SPECT-CT images. Subsequently, the semiquantitative uptake of 123I-MIBG in these VOIs was calculated [13].
Hyperinsulinaemic–euglycaemic clamp
The clamps were performed in the morning after an overnight fast. A cannula was inserted into the antecubital vein of the left arm. This cannula was used to infuse human soluble insulin (Actrapid; Novo Nordisk, Alphen aan den Rijn, the Netherlands) and 20% wt/vol. dextrose. A second cannula was inserted into a vein on the dorsum of the right hand, which was placed into a heated hand box to arterialise the venous blood for blood sampling [14]. Infusion of human insulin was started at 1.5 mU kg−1 min−1, and 20% dextrose solution was infused at a variable rate to achieve a blood glucose concentration of 5.0 mmol/l, which was maintained for 120 min. Plasma glucose concentrations were measured every 5 min at the bedside. In the last 30 min, blood samples were drawn at 10 min intervals for the measurement of insulin concentration. Insulin sensitivity was expressed as the mean glucose disposal rate (mg kg−1 min−1) during the last 30 min (i.e. the steady state) of the euglycaemic clamp.
Statistical analysis
The characteristics of the study participants are reported as medians with interquartile range (IQR). The p values for differences between South Asians and Europids in population characteristics, BAT volumes, SUV (mean and maximal) values of 18F-FDG, mean and maximal semiquantitative uptake value (SQUV) of 123I-MIBG and insulin sensitivity were determined with a Mann–Whitney U test. Spearman’s correlations between the SUVmax of 18F-FDG and both the SQUV of 123I-MIBG and insulin sensitivity were determined. Data analysis was performed using SPSS software 18.0 (Chicago, IL, USA). A p value <0.05 was considered statistically significant.
Results
Table 1 shows the characteristics of our study population after stratification by ethnicity. Apart from a higher FPG and a lower HDL-cholesterol, the characteristics of the South Asians were similar to those of the Europids.
Both in the Europids and the South Asians, 18F-FDG uptake in BAT was visually observed in eight out of ten male volunteers. Uptake of 123I-MIBG in BAT was observed in seven out of ten Europids, whereas six out of eight South Asians showed 123I-MIBG uptake in BAT (Fig. 1).
(a, e) BAT in a South-Asian participant visualised with 18F-FDG PET-CT and 123I-MIBG SPECT-CT (maximum-intensity-projection images). 18F-FDG and 123I-MIBG uptake on corresponding transversal PET and SPECT images (b, f) is suggestive of BAT and is superimposed on adipose tissue on correlated transversal CT images (c, d and g, h, respectively)
The median SUVmax of 18F-FDG (i.e. metabolic activity of BAT) was not significantly different between South Asians (7.5 [2.2–10.6] g/ml) and Europids (4.5 [2.2–8.4] g/ml) (p = 0.59 [Table 1]). Furthermore, there were no ethnic differences in median SUVmean of 18F-FDG (2.4 g/ml in both groups) or in median BAT volume (16.0 [5.5–64.5] cm3 in Europids, 38.4 [8.4–59.3] cm3 in South Asians; p = 0.57).
In addition, we found no significant difference between the ethnic groups for either the mean or the maximal SQUV (i.e. sympathetic stimulation of BAT) of 123I-MIBG (p = 0.26 for the mean and p = 0.41 for maximal SQUV of 123I-MIBG).
The correlation between the SQUV of 123I-MIBG and the SUVmax of 18F-FDG was positive, both in the total population (ρ = 0.80, p < 0.001) and after stratification by ethnic group (Europids: ρ = 0.65, p = 0.04; South Asians: ρ = 0.83, p = 0.01; Fig. 2).
The steady-state insulin levels of the Europids were similar to those of the South Asians (Table 1). There was no statistically significant difference in insulin sensitivity between the ethnic groups. Furthermore, there was no correlation between BAT activity and insulin sensitivity in the total population or after stratification by ethnicity (total group: ρ = −0.10, p = 0.68; Europids: ρ = 0.04, p = 0.91; South Asians: ρ = −0.14, p = 0.73).
Discussion
This is the first study that has prospectively investigated ethnic differences in metabolic BAT activity after cold exposure. We did not find differences in metabolic BAT activity or the sympathetic stimulation of BAT between South Asians and Europids. Therefore, it seems unlikely that BAT plays an important role in the development of the unfavourable metabolic profile in populations of South-Asian origin.
The ability to visualise metabolically active BAT in humans with 18F-FDG PET-CT under conditions of mild cold exposure has been reported in several studies [6, 8, 9, 15]. In our study, metabolically active BAT was found with 18F-FDG PET-CT in 80% of all participants after 2 h of cold exposure (16–18°C), which is in line with previous publications [8]. Furthermore, the median SUVmax of 18F-FDG in BAT is similar to values reported by Cypess et al, who also investigated BAT activity in lean, healthy men [16].
It is rather cumbersome to compare our data for SUVmean and BAT volume with the results of other studies, as the SUVmean and the derived BAT volume are largely dependent on the position and the definition of the ROI. As is to be expected in an emerging field of research, there is no consensus on the most appropriate way to define the ROI. In our study, a threshold for SUV of 18F-FDG of at least 2.0 g/ml was considered to indicate BAT (i.e. our ROI). Cypess et al used the same threshold [16]. However, Vosselman et al used a threshold of 1.5 g/ml [17] and van Marken Lichtenbelt et al reported that they used a ‘set threshold’ [8], whereas Carey et al and Ouellet et al considered an SUV >1.0 g/ml as BAT [18, 19]. The lower the chosen threshold, the higher the observed volume of BAT will be. For this reason, we cannot make direct comparisons between our results for SUVmean and BAT volume and those of other studies.
The majority of the published studies on BAT activity have focused on individuals of European origin. However, there are data available on BAT activity after cold exposure in those with other ethnic backgrounds. For example, Saito et al studied BAT activity in Japanese men and women [15], whereas Miao et al focused on Chinese individuals [20]. It should be realised, however, that individuals of Chinese and Japanese origins have different metabolic constitutions from those of South-Asian origin. For example, Karter et al found that type 2 diabetes prevalence was much higher in South Asians than in Chinese, Japanese and other, unspecified, Asians [21]. Moreover, type 2 diabetes seems to develop at an earlier age and at lower levels of BMI in South Asians than in other populations [22]. For these reasons, we specifically focused on the BAT activity in South Asians in our study.
This is the first study that has prospectively compared BAT activity between ethnic groups. We found that the metabolic activity of BAT was not different between South Asians and Europids. We did not expect this finding, as both the tendency of South Asians to develop abdominal obesity and type 2 diabetes [1, 2, 4] and the ontogenetic superfluity of the presence of BAT in the warm sub-Indian continent suggest BAT activity would be lower in South Asians than in Europids. As nine out of ten South Asians in our study were born in the Netherlands, it is unlikely that environmental factors influenced our results. Therefore, although surprising, our results suggest that the high risk of metabolic disturbance in South Asians, when compared with Europids, is not (partly) attributable to a relatively low BAT activity.
In line with the observed lack of ethnic difference in metabolic BAT activity, we did not find a difference in the sympathetic stimulation of BAT between the Europids and South Asians in our study. We previously validated 123I-MIBG SPECT-CT as a method to visualise and quantify the sympathetic stimulation of BAT in the ten Europid men who were also included in the present study [13]. In our previous study, we found that the correlation between the SQUV of 123I-MIBG and SUVmax of 18F-FDG was positive. The current finding of a positive correlation in South Asians in the present study corroborates our previous results.
Our study did not show a correlation between BAT activity and insulin sensitivity either in the total population or after stratification by ethnicity. Animal studies have shown that the BAT activity in both obese and insulin-resistant mice is lower than in healthy mice [12]. Furthermore, obese people have a lower BAT activity compared with their lean peers [8]. In our study, all the individuals included were healthy and the absolute mean glucose disposal rates were within the expected range. Prospective follow-up studies are needed to determine how the correlation between BAT activity and insulin resistance develops over time.
This study has limitations. Our sample size was small, so caution must be applied when extrapolating these results to the broader community. Furthermore, the limited number of participants may have resulted in a lack of power to demonstrate small differences between the ethnic groups. However, the mean SUVmax of 18F-FDG appeared higher in South Asians than in Europids. Therefore, it is unlikely that a greater sample size would alter our conclusion.
Furthermore, we did not perform a quantitative body-fat assessment in our study participants. For this reason, we cannot rule out the possibility that an ethnic difference in insulation, as a result of a difference in body-fat mass, has influenced our results on BAT activity. However, we did measure waist circumference and BMI, both of which have been shown to correlate well with total and regional measures of adiposity [23]. Given the low, lean values of BMI and waist circumference in both the South Asians and Europids in our study, it is not likely that the absolute difference in fat mass between the ethnic groups was great enough to have affected our results significantly. In addition, we used a room with a fixed temperature of 16–18°C to activate the BAT of our participants. This cooling method has been shown to be effective for the assessment of BAT activity in several other studies [8, 9, 15]. However, interindividual variation in the onset temperature of shivering has been reported, with some individuals starting to shiver at temperatures lower than 16–18°C [24]. As the thermogenesis of BAT is deemed to be at its highest just before the onset temperature of shivering [24], it is possible that BAT was not maximally activated in some of our study participants. As it is unknown whether the onset temperature of shivering differs between ethnic groups, it remains unclear whether our findings of a lack of ethnic differences in BAT activity were affected.
In conclusion, we found no difference in BAT activity during cold exposure between Europids and South Asians. Furthermore, we observed no ethnic differences in factors influencing BAT activity. Therefore, it seems unlikely that BAT plays an important role in the development of the unfavourable metabolic profile of populations of South-Asian origin.
Abbreviations
- BAT:
-
Brown adipose tissue
- CT:
-
Computed tomography
- 18F-FDG:
-
18F-fluorodeoxyglucose
- FPG:
-
Fasting plasma glucose
- 123I-MIBG:
-
123I-metaiodobenzylguanidine
- IQR:
-
Interquartile range
- PET:
-
Positron-emission tomography
- ROI:
-
Region of interest
- SPECT:
-
Single-photon emission computed tomography
- SQUV:
-
Semiquantitative uptake value
- SUVmax :
-
Maximal standardised uptake value
- SUVmean :
-
Mean standardised uptake value
- VOI:
-
Volume of interest
References
Chowdhury TA, Grace C, Kopelman PG (2003) Preventing diabetes in south Asians. BMJ 327:1059–1060
Gholap N, Davies M, Patel K, Sattar N, Khunti K (2011) Type 2 diabetes and cardiovascular disease in South Asians. Prim Care Diabetes 5:45–56
Oldroyd J, Banerjee M, Heald A, Cruickshank K (2005) Diabetes and ethnic minorities. Postgrad Med J 81:486–490
Samanta A, Burden AC, Fent B (1987) Comparative prevalence of non-insulin-dependent diabetes mellitus in Asian and white Caucasian adults. Diabetes Res Clin Pract 4:1–6
Whincup PH, Gilg JA, Papacosta O et al (2002) Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ 324:635
Cypess AM, Lehman S, Williams G et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
Nedergaard J, Bengtsson T, Cannon B (2007) Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293:E444–E452
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Virtanen KA, Lidell ME, Orava J et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
Cronin CG, Prakash P, Daniels GH et al (2012) Brown fat at PET/CT: correlation with patient characteristics. Radiology 263:836–842
Raji A, Gerhard-Herman MD, Warren M et al (2004) Insulin resistance and vascular dysfunction in nondiabetic Asian Indians. J Clin Endocrinol Metab 89:3965–3972
Enerback S (2010) Human brown adipose tissue. Cell Metab 11:248–252
Admiraal WM, Holleman F, Bahler L, Soeters MR, Hoekstra JB, Verberne HJ (2013) Combining 123I-metaiodobenzylguanidine SPECT/CT and 18F-FDG PET/CT for the assessment of brown adipose tissue activity in humans during cold exposure. J Nucl Med 54:208–212
Abumrad NN, Rabin D, Diamond MP, Lacy WW (1981) Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 30:936–940
Saito M, Okamatsu-Ogura Y, Matsushita M et al (2009) High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58:1526–1531
Cypess AM, Chen YC, Sze C et al (2012) Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A 109:10001–10005
Vosselman MJ, van der Lans AA, Brans B et al (2012) Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes 61:3106–3113
Carey AL, Formosa MF, van Every B et al (2013) Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia 56:147–155
Ouellet V, Routhier-Labadie A, Bellemare W et al (2011) Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab 96:192–199
Miao Q, Zhao XL, Zhang QY et al (2012) Stability in brain glucose metabolism following brown adipose tissue inactivation in Chinese adults. AJNR Am J Neuroradiol 33:1464–1469
Karter AJ, Schillinger D, Adams AS et al (2013) Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the Diabetes Study of Northern California (DISTANCE). Diabetes Care 36:574–579
Mangalmurti SS, Paley A, Gany F, Fisher EA, Hochman JS (2010) South Asians and risk of cardiovascular disease: current insights and trends. Ethn Dis 20:474–478
Flegal KM, Shepherd JA, Looker AC et al (2009) Comparisons of percentage body fat, body mass index, waist circumference, and waist-stature ratio in adults. Am J Clin Nutr 89:500–508
Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD (2011) Brown adipose tissue in morbidly obese subjects. PLoS One 6:e17247
Acknowledgement
The results from this study were presented as an abstract at the ADA 73rd Scientific Sessions in June 2013.
Duality of interest
All authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
WMA contributed to the design of the study, collected, analysed and interpreted the data and drafted the manuscript. HJV contributed to the design of the study, analysis of the data, and reviewed and edited the manuscript. FAK collected data, and reviewed and edited the manuscript. FH contributed to the design of the study and interpretation of data analysis, and reviewed and edited the manuscript. MRS contributed to the design of the study and reviewed and edited the manuscript. JBLH contributed to the design of the study and edited the manuscript. All of the authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Admiraal, W.M., Verberne, H.J., Karamat, F.A. et al. Cold-induced activity of brown adipose tissue in young lean men of South-Asian and European origin. Diabetologia 56, 2231–2237 (2013). https://doi.org/10.1007/s00125-013-2938-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2938-5