Abstract
Aims/hypothesis
We examined the physiological mechanisms by which cannabinoid receptor 1 (CB1) antagonism improves glucose metabolism and insulin sensitivity independent of its anorectic and weight-reducing effects, as well as the effects of CB1 antagonism on brown adipose tissue (BAT) function.
Methods
Three groups of diet-induced obese mice received for 1 month: vehicle; the selective CB1 antagonist SR141716; or vehicle/pair-feeding. After measurements of body composition and energy expenditure, mice underwent euglycaemic–hyperinsulinaemic clamp studies to assess in vivo insulin action. In separate cohorts, we assessed insulin action in weight-reduced mice with diet-induced obesity (DIO), and the effect of CB1 antagonism on BAT thermogenesis. Surgical denervation of interscapular BAT (iBAT) was carried out in order to study the requirement for the sympathetic nervous system in mediating the effects of CB1 antagonism on BAT function.
Results
Weight loss associated with chronic CB1 antagonism was accompanied by increased energy expenditure, enhanced insulin-stimulated glucose utilisation, and marked activation of BAT thermogenesis. Insulin-dependent glucose uptake was significantly increased in white adipose tissue and BAT, whereas glycogen synthesis was increased in liver, fat and muscle. Despite marked weight loss in the mice, SR141716 treatment did not improve insulin-mediated suppression of hepatic glucose production nor increase skeletal muscle glucose uptake. Denervation of iBAT blunted the effect of SR141716 on iBAT differentiation and insulin-mediated glucose uptake.
Conclusions/interpretation
Chronic CB1 antagonism markedly enhances insulin-mediated glucose utilisation in DIO mice, independent of its anorectic and weight-reducing effects. The potent effect on insulin-stimulated BAT glucose uptake reveals a novel role for CB1 receptors as regulators of glucose metabolism.
Similar content being viewed by others
Introduction
The endocannabinoid system has emerged as a key player in both the central and peripheral control of energy balance and glucose metabolism. Chronic pharmacological interventions with selective inhibitors of cannabinoid receptor 1 (CB1), such as rimonabant (SR141716), leads to decreased adiposity as the result of both reduced food intake and increased energy expenditure. The latter mechanism plays a larger role in the weight-loss effects of these drugs, as demonstrated by pair-feeding studies [1, 2]. Mice null for Cb1 (also known as Cnr1) display reduced body weight, adiposity, resistance to diet-induced obesity (DIO), and improvement in glucose regulation [3]. These results implicate the CB1 receptors and its intracellular signalling as critical modulators of energy balance and glucose metabolism.
Despite clear evidence that pharmacological CB1 antagonism improves insulin sensitivity [1, 4–7], the specific mechanisms are still under investigation. In particular, it is still unclear how chronic CB1 antagonism improves insulin sensitivity and what tissues are its target of action. In this regard, the brown adipose tissue (BAT) has emerged as an important modulator of energy expenditure and glucose utilisation [8, 9]. The notion that adult humans do not retain functional BAT has been dispelled by recent evidence, which points to BAT as an important player in the pathogenesis of obesity and diabetes [10–12]. Notably, pharmacological blockade or genetic ablation of CB1 has been linked to the activation of the BAT, which further underscores the role of this tissue as a potential target for obesity and diabetes therapy [8].
In this report, we examine the effects of CB1 antagonism on glucose metabolism and insulin sensitivity, independent of its effects on food intake and adiposity. Since CB1 antagonism activates BAT thermogenesis and glucose uptake, we investigated whether these effects require an intact sympathetic nervous system (SNS).
Methods
Mice
All animal protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee. Nine-week-old C57BL/6 J male mice (Jackson Laboratories, Bar Harbor, ME, USA) were housed in temperature-controlled rooms (21–23°C) with a 12 h light–dark cycle, and fed with a 60% high-fat diet (D12492, Research Diets, New Brunswick, NJ, USA) for 1 month.
Energy balance and euglycaemic–hyperinsulinaemic clamp studies
DIO mice were randomised into three groups (n = 11/group): vehicle-treated with unlimited access to food (VEH); SR141716-treated with unlimited access to food (SR); and vehicle-treated and pair-fed with the average amount of food ingested by the SR group during the previous day (PF). Mice were injected daily i.p. and fed 1 h before lights out for 7 weeks. Body composition by quantitative magnetic resonance (Echo MRI Whole Body Composition Analyzer, Echo Medical Systems, Houston, TX, USA) and indirect calorimetry (TSE Systems, Chesterfield, MO, USA) were performed after 4 weeks of treatment. Euglycaemic–hyperinsulinaemic clamp experiments were carried out after 5–6 weeks of treatment.
BAT denervation studies
The interscapular BAT (iBAT) of DIO mice was surgically denervated or sham-operated. After recovery, each group was randomised to receive SR or vehicle (n = 13–15/group). Mice were injected daily i.p. 1 h before lights out for 8 weeks. All mice had unlimited access to food and water. Food intake and body weight were recorded daily. Body composition analysis by MRI and indirect calorimetry were performed after 6 weeks of treatment and glucose uptake after 8–9 weeks of treatment.
BAT thermogenesis studies
DIO mice were implanted with temperature probes (see methods in the electronic supplementary material [ESM]) or sham-operated. After recovery, the mice received vehicle or SR (n = 6/group). Mice were injected i.p. daily 4 h before lights out. BAT temperature was measured every 30 min from 11:00 hours to 19:00 hours for 12 days. Afterwards, mice were implanted with a jugular catheter and, upon recovery, were injected with a bolus of 2-deoxy-d-[U-14 C]glucose (370 kBq) to measure basal glucose uptake in BAT. All mice had unlimited access to food and water.
Food-restricted euglycaemic–hyperinsulinaemic clamp studies
Ten-week-old C57bl/6 mice from Jackson Laboratories were fed a low-fat standard chow or a 60% high-fat diet (D12492, Research Diets) for 5–6 weeks. DIO mice were divided into two groups receiving a high-fat diet that was either unlimited or restricted to 85% of the unlimited food intake. After 5 weeks, mice underwent surgical implantation of jugular catheters and, after recovery, underwent euglycaemic–hyperinsulinaemic clamp studies (n = 5/group).
Drug studies
SR141716 was obtained from the NIMH Chemical Synthesis and Drug Supply Program (www.nimh-repository.rti.org), and administered i.p. at 10 mg/kg daily in all studies. This dose has been previously used i.p. and by mouth to similar effect [9, 13, 14]. A 1 mg/ml emulsion of SR141716 was made by dissolving 100 mg of SR141716 in 1 ml DMSO. Next, 2 ml of Tween 80 was added while vortexing. Finally, 97 ml of saline was slowly added while vortexing.
Euglycaemic–hyperinsulinaemic clamp studies and glucose uptake in skeletal muscle
Euglycaemic–hyperinsulinaemic clamp studies were performed in conscious, chronically catheterised mice, as described by Okamoto et al. and Lo et al. [15, 16]. Briefly, during the clamp studies, which lasted for 90 min, we infused a solution of glucose (20% wt/vol.) at a variable rate as required to maintain euglycaemia. Mice received primed-constant infusions of [3-3H]glucose (bolus of 74 kBq followed by 3.7 kBq/min; NET-331 C, Perkin Elmer, Boston, MA, USA) and insulin (bolus of 88 mU/kg followed by 5 mU min−1 kg−1; Humulin R, Eli Lilly, Indianapolis, IN, USA).
We collected plasma samples to determine glucose concentrations and tracer-specific activities at times 0, 30, 50, 60, 70, 80 and 90 min. Additional sample volumes were taken at 0, 60 and 90 min to determine basal and clamped insulin levels and NEFA. To measure tissue-specific glucose uptake, we injected a bolus (370 kBq) of 2-deoxy-d-[U-14 C]glucose at time = 45 min. At the end of the clamp studies, mice were killed and tissue samples were quickly harvested and stored at −80°C for further analysis.
Indirect calorimetry
Indirect calorimetry was performed as described by Pfluger et al. [17]. Mice were individually housed in metabolic chambers (TSE Systems) in which fluid, food intake, locomotor activity and gas exchanges can be monitored. Following 48 h of acclimatisation, O2 consumption, CO2 production and locomotor activity were measured every 6 min for a total of 72 h to measure the gas exchange, respiratory quotient and energy expenditure. Energy expenditure values were normalised to the body weight of the animals. Home-cage locomotor activity was determined using a tri-dimensional infrared light beam system and expressed as beam breaks/24 h.
Western blot
Tissue was homogenised using the Qiagen TissueLyser bead mill (Qiagen, Valencia, CA, USA). Proteins were extracted with RIPA buffer (150 mmol/l NaCl, 1.0% [vol./vol.] Triton X-100, 0.5% [wt/vol.] sodium deoxycholate, 0.1% [vol./vol.] SDS, 50 mmol/l Tris, pH 8.0) and then centrifuged for 10 min at 10,000 g. A 30 μg sample of protein was separated on a 10% Tris-HCl gel and transferred to polyvinylidene-fluoride membrane for 1 h at 4°C at 100 V. The membrane was blocked with 5% (wt/vol.) non-fat dry milk in Tris-buffered saline with Tween 20 for 1 h at room temperature, followed by 1:1,000 dilution of tyrosine hydroxylase antibody (catalogue identifier ab152, Millipore, Billerica, MA, USA) overnight at 4°C with a 1:10,000 dilution of goat anti-rabbit secondary antibody (Abcam, Cambridge, MA, USA) for 1 h at room temperature. Bands were visualised using the Pierce ECL western blotting substrate (catalogue identifier 32106, Thermo Scientific, Rockford, IL, USA).
Analytical procedures and calculations
Plasma glucose was measured by the glucose oxidase method in a GM7 Analyser (Analox Instruments USA, Lunenburg, MA, USA). The rate of glycolysis was determined by measuring the tritium on the C-3 position of our glucose tracer that is lost into water during glycolysis [18]. Thus, the plasma tritium is present in two chemical forms: 3H-labelled water or [3-3H]glucose. To measure both forms, we counted radioactivity in plasma samples deproteinised with Ba(OH)2 and ZnSO4, before and after each sample was evaporated to dryness. The dry counts represent plasma [3-3H]glucose, whereas the difference between the wet and dry counts is a measure of the tritiated water.
Under steady-state conditions for plasma glucose, the rate of glucose disposal (R d) is assumed to be equal to the rate of glucose appearance (R a). We determined R d by dividing the infusion rate for [3-3H]glucose (disintegrations/min) by the specific activity of plasma [3-3H]glucose (disintegrations min–1 [mg glucose]–1). The rate of glucose production is calculated as the difference between R d and the rate of glucose infusion.
The tissue-specific rate of glycogen synthesis was quantified by measuring the rate of incorporation of [3-3H]glucose into glycogen. Tissue glycogen concentrations were determined after digestion with amyloglucosidase, as described by Massillon et al. and Rossetti et al. [18, 19]. The glycogen synthetic rate was obtained by dividing the [3-3H]glucose radioactivity in glycogen (disintegrations min–1 [g tissue]–1) by the mean specific activity of [3-3H]glucose in plasma during the insulin clamp (disintegrations min–1 [μg plasma glucose]–1).
To measure the tissue-specific rate of glucose uptake we weighed and dissolved tissues in 0.5 ml of 1 mol/l NaOH, and incubated them in a shaking water bath at 60°C for 1 h. After neutralisation with 0.5 ml of 1 mol/l HCl, two aliquots were taken. One was deproteinised with Ba(OH)2 and ZnSO4 and the other with a 6% solution of HClO4. The HClO4 supernatant fraction contained both phosphorylated and unphosphorylated 2-deoxyglucose, whereas the Ba(OH)2 and ZnSO4 supernatant fraction contained only the unphosphorylated form. The difference in counts between the two supernatant fractions is a measure of the tissue content of 2-deoxy-[U-14 C]glucose 6-phosphate.
The rate of tissue glucose uptake was obtained by dividing the tissue content of 2-deoxy-[U-14 C]glucose 6-phosphate by the specific activity of 2-deoxy-[U-14 C]glucose in plasma [19]. Plasma insulin and leptin were measured with a MILLIPLEX MAP Mouse Serum Adipokine Panel (catalogue identifier MADPK-71 K, Linco Research, St Charles, MO, USA). Plasma adiponectin was measured with MILLIPLEX MAP Mouse Adipocyte Panel (catalogue identifier MADPCYT-72 K, Millipore). NEFA in plasma was measured with a kit from Waco Pure Chemical Industries (Osaka, Japan).
iBAT denervation
Mice were anaesthetised with isofluorane and a transverse incision was made in the skin anterior to the iBAT fat pads. The pads were identified and separated from the underlying muscle by blunt dissection. Four intercostal nerve bundles entering each fat pad were identified, and a section of each nerve bundle was removed bilaterally. Extra care was taken to ensure that the blood supply was not disrupted.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, La Jolla, CA, USA). One-way ANOVA with Bonferroni post hoc test was used to determine significance at (p ≤ 0.05) for studies involving pair-feeding. Denervation studies were analysed using a two-way ANOVA with Bonferroni post hoc test for significance (p ≤ 0.05). All time course studies were analysed by two-way ANOVA with repeated measures and Bonferroni post hoc test (p ≤ 0.05).
Results
CB1 antagonism reduces adiposity and increases energy expenditure in DIO mice
To determine the effects of chronic CB1 antagonism on energy balance and body composition, DIO mice received daily i.p. injections of SR141716 for 4 weeks or its vehicle, or were pair fed according to the intake of the SR group for the same length of time. SR treatment elicited a profound but transient reduction in food intake (Fig. 1a), which is consistent with the rapid weight loss achieved in the first few days of SR treatment and pair-feeding [1, 2]. However, pair-feeding alone was insufficient to attain the same weight loss as the SR group (Fig. 1b). The fat mass of DIO mice was significantly reduced in the SR group compared with the VEH and PF groups, suggesting that the loss of adiposity with CB1 antagonism is mediated by increased energy expenditure (Fig. 1c, d). Indeed, energy expenditure measured by indirect calorimetry was increased in the SR group compared with both VEH and PF groups (Fig. 1e), and was not due to increased locomotor activity (Fig. 1f). As expected in DIO mice consuming a high-fat diet, the respiratory quotient was very low and unchanged in all groups (VEH, 0.75 ± 0.008; PF, 0.74 ± 0.008; SR, 0.74 ± 0.008). Consistent with the changes in fat mass, plasma leptin levels were significantly decreased by SR141716 treatment, but not by pair-feeding (Table 1).
Energy balance and body composition in DIO C57BL/6 mice treated with 10 mg/kg SR i.p. or vehicle. After 4 weeks of treatment mice were moved to indirect calorimetry cages for 4 days. a Food intake and (b) body weight of VEH (white squares), PF (triangles) and SR (black squares) mice. c Fat mass and (d) percentage change in fat mass from baseline of VEH (white bars), PF (hatched bars) and SR (black bars) mice measured by MRI. e Energy expenditure and (f) locomotor activity were measured by indirect calorimetry cages equipped with a beam-break measurement system. In all groups, n = 11; *p < 0.05 SR group vs PF and VEH groups; † p < 0.05 SR group vs VEH group only
CB1 antagonism increases whole-body insulin-dependent glucose utilisation
SR-induced weight loss was accompanied by a reduction in fasting plasma glucose and insulin levels (Table 1), suggesting improved insulin sensitivity. Fasting levels of adiponectin, triacylglycerols and NEFA were not changed. However, insulin-dependent suppression of plasma NEFA was restored selectively in the SR group (Table 1).
To determine the effects of CB1 antagonism on in vivo insulin action independent of food intake, we performed euglycaemic–hyperinsulinaemic clamp studies in the SR, VEH and PF groups. Glucose infusion rate was significantly higher in the SR group compared with the VEH or PF groups, indicating that CB1 antagonism significantly improves whole-body insulin sensitivity (Fig. 2a). This effect was due to an increase in glucose disposal rate (Fig. 2b). However, the rate of glucose production was not changed among the three groups (Fig. 2c), and neither was the expression of the genes encoding the hepatic gluconeogenic enzymes phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (data not shown), indicating that weight loss induced by SR141716 does not improve hepatic insulin resistance of DIO mice. Consistent with an increased glucose utilisation, SR141716 increased the whole-body glycolysis rate (Fig. 2d).
Hyperinsulinaemic–euglycaemic clamp studies. After 7 weeks of treatment with VEH (white bars), PF (hatched bars) and SR (black bars), mice underwent a hyperinsulinaemic–euglycaemic clamp. Graphs show: (a) glucose infusion rate; (b) glucose disposal rate; (c) glucose production rate; (d) whole-body glycolysis rate. Glucose uptake was measured by 2-deoxy-[U-14 C]glucose in WAT (e) and iBAT (f). In all groups, n = 11. *p < 0.05 SR group vs PF and VEH groups; † p < 0.05 SR group vs VEH group only
In order to identify the target tissues for an SR141716-induced increase in glucose utilisation, we measured in vivo glucose uptake in fat and skeletal muscle under hyperinsulinaemic conditions. Glucose uptake in white adipose tissue (WAT; Fig. 2e) and BAT (Fig. 2f) was significantly stimulated by SR141716. In particular, CB1 antagonism stimulated the uptake of glucose in BAT by more than threefold in the SR group compared with the VEH and PF groups (Fig. 2f). In a separate cohort of mice treated with SR141716 or vehicle, we measured in vivo BAT glucose uptake under basal conditions and found no significant difference (31.9 ± 3.9 mg kg−1 min−1 and 45.1 ± 7.0 mg kg−1 min−1 for the VEH and SR groups, respectively; n = 6/group, p > 0.05). The lack of an increase in BAT glucose uptake under basal conditions suggests that the effect of SR141716 on BAT glucose uptake is mediated by improved insulin sensitivity.
Despite the marked increase in whole-body glucose uptake, the SR group did not display a significant increase in the rate of insulin-stimulated glucose uptake in skeletal muscle (Fig. 3a). However, the rate of insulin-dependent glycogen synthesis was markedly and significantly elevated in skeletal muscle as well as in WAT and liver (Fig. 3b). Notably, pair-feeding was insufficient to elicit any improvement in insulin-dependent glucose metabolism (Figs 2 and 3).
Rate of tissue glucose uptake and glycogen synthesis. a Glucose uptake in several muscles measured by 2-deoxy-[U-14 C]glucose. b Glycogen synthesis rate in several muscles, liver and WAT in VEH (white bars), PF (hatched bars) and SR (black bars) groups. In all groups, n = 11. EDL, extensor digitorum longus; Gast, gastrocnemius; Sol, soleus. *p < 0.05 SR group vs PF and VEH groups; † p < 0.05 SR group vs VEH group only
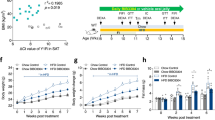
To determine whether chronic CB1 antagonism improved insulin sensitivity via its effect on fat mass reduction, we induced weight loss in DIO mice by food restriction to match the weight reduction achieved with SR141716. We then subjected these mice to euglycaemic–hyperinsulinaemic clamp studies (ESM Fig. 1). Despite reduced adiposity, food-restricted DIO mice did not have improved insulin sensitivity compared with DIO mice that had unrestricted access to food (ESM Fig. 1). Thus, CB1 antagonism improves insulin sensitivity independent of its ability to reduce adiposity.
CB1 antagonism activates BAT
The marked increase in glucose utilisation in BAT suggested that CB1 antagonism could exert weight-reducing and insulin-sensitising effects via stimulation of BAT function. Therefore, we tested the ability of SR141716 to activate the BAT function of DIO mice by measuring the temperature of iBAT pads using thermal probes [20]. Temperature-sensing transponders were surgically implanted underneath the iBAT of DIO mice. After recovery, baseline BAT temperatures were recorded every 30 min or hourly from 11:00 to 19:00 hours, across the transition from light to dark in the light–dark cycle (Fig. 4a–c). Then, mice were randomised to receive a daily dose of SR141716 or vehicle.
SR141716 treatment induces BAT thermogenesis. iBAT temperature measurement with implantable probes during (a) the first 3 days and (b) the last 3 days of treatment with 10 mg/kg SR141716 i.p. (black squares) or vehicle (white squares). The shaded area indicates the dark period, while the vertical line indicates time of injection. c AUCs of iBAT temperature during days 1–3 and 10–12 in VEH (white bars) and SR (black bars). d Expression of Pgc-1α, Ucp1 and Pparδ in iBAT from VEH (white bars), SR (black bars) and PF (hatched bars) groups. For a–c, n = 6, and for d, n = 11; *p < 0.05 (SR group vs other groups on the same graph). a.u., arbitrary units
Baseline BAT temperatures were similar in both groups and displayed the typical circadian profile with higher BAT temperatures in the dark period (ESM Fig. 2). SR141716 injection promptly increased BAT temperature within 1 h from the first dose (Fig. 4a) compared with the same time at baseline or with vehicle (average increase of 1.5°C). VEH mice showed no change in BAT temperature compared with baseline. The difference in iBAT temperature between the SR and VEH groups was greater in the light than in the dark phase of the cycle, perhaps because of the time of SR141716 delivery (12:00 hours in the light cycle). Although the magnitude of SR141716 effect on iBAT temperature was attenuated by day 10 (Fig. 4b), the AUC of iBAT temperature for days 10 to 12 was still significantly elevated (Fig. 4c).
The strong thermogenic effect of SR141716 on BAT was accompanied by a significant increase in BAT expression of Ucp1, which encodes the uncoupling protein that mediates thermogenesis in BAT [21], and Pgc-1α (also known as Ppargc1a) and Pparδ (also known as Ppard), which encode the co-activator and transcription factor, respectively, that are implicated in the activation and differentiation of BAT [22] (Fig. 4d).
In a separate cohort, we examined the morphological changes induced by SR141716 in BAT (Fig. 5a,b). CB1 antagonism prominently increased the number of multilocular adipocytes, a typical feature of activated BAT. Taken together these data indicate that CB1 antagonism causes a marked activation of BAT function.
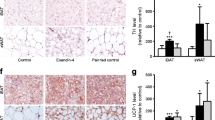
Effect of CB1 antagonism on iBAT morphology in sham-operated or denervated mice. a–d Haematoxylin and eosin staining of iBAT after chronic treatment with 10 mg/kg SR (b,d) or vehicle (a,c) in sham-operated (a,b) and denervated (c,d) mice. In order to confirm effectiveness of the surgical denervation, tyrosine hydroxylase, a marker of SNS innervation, was measured by western blot (e). In all groups, n = 4. D-VEH, denervated VEH group; D-SR, denervated SR group; S-SR, sham-operated SR group; S-VEH, sham-operated VEH group; β-Tub, β-tubulin; TH, tyrosine hydroxylase
SNS-dependent effects of CB1 antagonism on iBAT
To determine whether the effects of CB1 antagonism on BAT require the SNS, we surgically denervated the iBAT of DIO mice and tested the effect of CB1 antagonism on weight loss and BAT function. Successful denervation was verified by immunoblot (Fig. 5e) and immunohistochemistry (ESM Fig. 3a-f) using tyrosine hydroxylase as a marker of sympathetic nerve endings. Denervation dramatically altered BAT morphology, turning its lipid droplets from multilocular to unilocular (Fig. 5c), and SR141716 treatment did not significantly reverse this effect (Fig. 5d). Denervation of iBAT had no effect on body weight or fat mass in VEH or SR groups (Fig. 6a,b). However, denervation blunted the ability of SR141716 to increase insulin-stimulated glucose uptake (Fig. 6c). The expression of Ucp1 was markedly downregulated by denervation and showed only a trend to be induced by SR independently of the SNS (Fig. 6d). On the other hand, Pgc-1α levels were not regulated by denervation, which blunted the ability of SR to induce Pgc-1α (Fig. 6e).
Effect of denervation on body weight, adiposity and iBAT glucose uptake and gene expression. a Body weight of SR and VEH animals (black and white symbols, respectively) that had sham operation or iBAT denervation (squares and triangles, respectively). In b–e, white bars and black bars represent VEH and SR groups, respectively. b Fat mass of SR and VEH animals that had sham operation or iBAT denervation. c–e Activation of iBAT in sham-operated and denervated animals after chronic treatment with SR141716. c Glucose uptake in iBAT measured by 2-deoxy-[U-14 C]glucose under hyperinsulinaemic conditions. d Ucp1mRNA expression (by two-way ANOVA: main effect of SR and denervation p ≤ 0.05). e Pgc-1α mRNA expression. For a and b, n = 13/group; for c, n = 8/group; and for d and e, n = 5/group; *p < 0.05 SR group vs VEH group. a.u., arbitrary units; Denerv, denervated
Discussion
In agreement with previous reports, we find that chronic CB1 antagonism reduces body weight because of both transient anorexia and, more importantly, increased energy expenditure [2].
Our data show that chronic SR141716 administration results in a significant increase in insulin-dependent glucose utilisation, including an increase in the rates of glycolysis and glycogen synthesis. In our experimental model of DIO, the effects of CB1 antagonism show an unexpected pattern in the amelioration of specific components of insulin action. Although SR141716 treatment greatly increased insulin-dependent glucose utilisation, the rate of hepatic glucose production was not significantly changed, implying that CB1 antagonism cannot improve hepatic insulin resistance in DIO mice. This result is at odds with previous reports in which chronic SR141716 treatment elicited a significant improvement in hepatic insulin sensitivity [4, 5]. The effect of SR141716 on hepatic insulin sensitivity was shown to depend on increased levels of plasma adiponectin, whereas the effects of SR141716 on weight loss and glucose utilisation were not [5]. Indeed, several reports have implicated the endocannabinoid system as a modulator of adiponectin production and secretion [4, 5, 23–26]. This adipokine is known as a powerful inhibitor of hepatic glucose production [27, 28]. However, we and others could not detect changes in adiponectin levels (Table 1) [1, 29–31], which might explain why we did not detect an improvement in hepatic insulin sensitivity. The reasons for this lack of effect on adiponectin are not known and need further investigation.
An alternative explanation for the failure of SR141716 to decrease hepatic glucose production could be an inhibitory effect of dietary fat on insulin sensitivity. Indeed, we show that weight reduction by food restriction alone in our DIO mice fails to ameliorate in vivo insulin action, albeit these mice have a fat mass similar to that of SR141716-treated DIO mice (ESM Fig. 1). These results also suggest that the stimulatory effects of SR141716 on insulin-dependent glucose utilisation are specific to weight loss secondary to CB1 antagonism, as similar weight loss induced with food restriction does not restore insulin sensitivity (ESM Fig. 1). Since weight reduction does not always appear to lower endocannabinoid levels in obese individuals [32], we speculate that the beneficial effects of SR141716 on glucose metabolism are possibly secondary to the blockade of overstimulated CB1 receptors.
Another typical result of weight loss is an improvement in insulin-stimulated glucose uptake in skeletal muscle [33], which was not observed in our experiments with chronic administration of SR141716, nor was it observed in the food-restricted DIO mice. Our findings are in agreement with previous reports that did not observe a significant effect of SR141716 on insulin-stimulated glucose uptake in muscle of DIO mice [4, 5] However, our studies did show significantly enhanced insulin-stimulated glycogen synthesis in skeletal muscle to an extent large enough to contribute significantly to the whole-body increase in glucose utilisation promoted by SR141716.
Notably, glycogen synthesis of skeletal muscle was increased in the absence of a significant stimulation of glucose uptake. Although the effect of insulin on glycogen stores is typically influenced by glucose transport, insulin can increase glycogen synthesis in muscle independently of an increase in glucose uptake [34]. The effect of SR141716 on glycogen synthesis is not mediated by a change in glycogen phosphorylase or glycogen synthase (data not shown). Thus, CB1 antagonism may affect the activity of glycogen enzymes via alteration of transduction signalling or production of glycogen regulatory proteins. One possible explanation for the lack of a significant increase in glucose uptake in muscle is that CB1 antagonism increases fatty acid oxidation [6] which, in turn, can lead to inhibition of glucose uptake [35].
Unlike muscle, chronic CB1 antagonism strikingly increases insulin-stimulated glucose uptake of BAT. However, under basal conditions, chronic CB1 antagonism does not lead to an appreciable increase in BAT glucose uptake. This result indicates that chronic CB1 antagonism does not increase basal glucose uptake in BAT, suggesting that an SNS-dependent increase in BAT glucose uptake is not tonically stimulated. However, it reveals a novel role for CB1 receptors in the SNS as modulators of insulin action in BAT.
Denervation studies show that CB1-antagonist-mediated BAT differentiation and activation are strongly dependent on SNS innervation. Our data are in agreement with a report showing that CB1 antagonism in lean rats activates BAT in an SNS-dependent fashion [8]. Moreover, Quarta et al. have shown that BAT denervation blunts the enhancement of BAT glucose uptake after administration of SR141716 or exposure to cold [9]. Accordingly, we observed changes in histological, molecular and thermogenic variables after surgical denervation that indicate a substantial loss of function of BAT.
Interestingly, although the effects of SR141716 on BAT functions were blunted, iBAT denervation did not alter SR141716-mediated loss of body weight or fat mass, or energy expenditure. Previous studies on denervation or ablation of iBAT have yielded contrasting results with failure [36–38] and success [37] in altering body weight. Explanations for the failure are that the denervation of iBAT is limited to only 40% of total BAT mass. This may lead to compensatory activation of BAT in other fat depots, particularly as our studies were not conducted at thermoneutrality (ambient temperature 22°C). Nevertheless, these results point to a novel role for CB1 receptors in modulating BAT insulin sensitivity with SNS-dependent mechanisms. Although the complex interaction between the SNS and insulin action needs to be elucidated, we can speculate that CB1 signalling through the SNS is crucial for the modulation of BAT glucose and fat uptake. Indeed, the coordinated partitioning of these fuels plays an important role in BAT thermogenesis [39, 40].
It is important to note that the CB1 receptors responsible for modulating BAT function and thermogenesis may not be exclusively located in the central nervous system. In support of this notion, peripheral but not central administration of SR141716 induced food-independent weight loss in DIO rats [6]. Also, peripherally restricted CB1 antagonists can cause substantial weight loss and increase energy expenditure [41]. Taken together, these data suggest the thermogenic effects of CB1 antagonism could involve peripheral mechanisms, including BAT activation.
In conclusion, our data show that CB1 antagonism markedly improves insulin-mediated glucose utilisation in DIO mice independent of its anorectic properties, via mechanisms that are not reproduced by food restriction alone. BAT is the major target organ for the effect of CB1 antagonism on insulin-dependent glucose uptake via a mechanism that requires an intact SNS. These novel data point to a crucial role of SNS CB1 receptors in modulating body weight and glucose metabolism through activation of BAT function.
Abbreviations
- CB1:
-
Cannabinoid receptor 1
- BAT:
-
Brown adipose tissue
- DIO:
-
Diet-induced obesity
- iBAT:
-
Interscapular BAT
- PF:
-
Vehicle-treated and pair-fed with the average amount of food ingested by the SR group during the previous day
- SNS:
-
Sympathetic nervous system
- SR:
-
SR141716-treated with unlimited access to food
- VEH:
-
Vehicle-treated with unlimited access to food
- WAT:
-
White adipose tissue
References
Cota D, Sandoval DA, Olivieri M et al (2009) Food intake-independent effects of CB1 antagonism on glucose and lipid metabolism. Obesity (Silver Spring) 17:1641–1645
Herling AW, Kilp S, Elvert R, Haschke G, Kramer W (2008) Increased energy expenditure contributes more to the body weight-reducing effect of rimonabant than reduced food intake in candy-fed wistar rats. Endocrinology 149:2557–2566
Ravinet TC, Delgorge C, Menet C, Arnone M, Soubrie P (2004) CB1 cannabinoid receptor knockout in mice leads to leanness, resistance to diet-induced obesity and enhanced leptin sensitivity. Int J Obes Relat Metab Disord 28:640–648
Migrenne S, Lacombe A, Lefevre AL et al (2009) Adiponectin is required to mediate rimonabant-induced improvement of insulin sensitivity but not body weight loss in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol 296:R929–R935
Watanabe T, Kubota N, Ohsugi M et al (2009) Rimonabant ameliorates insulin resistance via both adiponectin-dependent and adiponectin-independent pathways. J Biol Chem 284:1803–1812
Nogueiras R, Veyrat-Durebex C, Suchanek PM et al (2008) Peripheral, but not central, CB1 antagonism provides food intake-independent metabolic benefits in diet-induced obese rats. Diabetes 57:2977–2991
O’Hare JD, Zielinski E, Cheng B, Scherer T, Buettner C (2011) Central endocannabinoid signaling regulates hepatic glucose production and systemic lipolysis. Diabetes 60:1055–1062
Verty AN, Allen AM, Oldfield BJ (2009) The effects of rimonabant on brown adipose tissue in rat: implications for energy expenditure. Obesity (Silver Spring) 17:254–261
Quarta C, Bellocchio L, Mancini G et al (2010) CB(1) signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell Metab 11:273–285
Virtanen KA, Lidell ME, Orava J et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
Cypess AM, Lehman S, Williams G et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Liu YL, Connoley IP, Wilson CA, Stock MJ (2005) Effects of the cannabinoid CB1 receptor antagonist SR141716 on oxygen consumption and soleus muscle glucose uptake in Lep(ob)/Lep(ob) mice. Int J Obes (Lond) 29:183–187
Rinaldi-Carmona M, Barth F, Heaulme M et al (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett 350:240–244
Okamoto H, Obici S, Accili D, Rossetti L (2005) Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest 115:1314–1322
Lo CM, Obici S, Dong HH et al (2011) Impaired insulin secretion and enhanced insulin sensitivity in cholecystokinin-deficient mice. Diabetes 60:2000–2007
Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA 105:9793–9798
Massillon D, Chen W, Hawkins M, Liu R, Barzilai N, Rossetti L (1995) Quantitation of hepatic glucose fluxes and pathways of hepatic glycogen synthesis in conscious mice. Am J Physiol 269:E1037–E1043
Rossetti L, Stenbit AE, Chen W et al (1997) Peripheral but not hepatic insulin resistance in mice with one disrupted allele of the glucose transporter type 4 (GLUT4) gene. J Clin Invest 100:1831–1839
Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ (2007) Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148:5339–5347
Enerback S, Jacobsson A, Simpson EM et al (1997) Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387:90–94
Kajimura S, Seale P, Spiegelman BM (2010) Transcriptional control of brown fat development. Cell Metabolism 11:257–262
Despres JP, van Gaal L, Pi-Sunyer X, Scheen A (2008) Efficacy and safety of the weight-loss drug rimonabant. Lancet 371:555–557
Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P (2010) CB1 antagonism exerts specific molecular effects on visceral and subcutaneous fat and reverses liver steatosis in diet-induced obese mice. Diabetes 59:926–934
Poirier B, Bidouard JP, Cadrouvele C et al (2005) The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab 7:65–72
Flamment M, Gueguen N, Wetterwald C, Simard G, Malthiery Y, Ducluzeau PH (2009) Effects of the cannabinoid CB1 antagonist, rimonabant, on hepatic mitochondrial function in rats fed a high fat diet. Am J Physiol Endocrinol Metab 297:E1004–E1012
Berg AH, Combs TP, Scherer PE (2002) ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab 13:84–89
Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108:1875–1881
Pagano C, Pilon C, Calcagno A et al (2007) The endogenous cannabinoid system stimulates glucose uptake in human fat cells via phosphatidylinositol 3-kinase and calcium-dependent mechanisms. J Clin Endocrinol Metab 92:4810–4819
Thornton-Jones ZD, Kennett GA, Benwell KR et al (2006) The cannabinoid CB1 receptor inverse agonist, rimonabant, modifies body weight and adiponectin function in diet-induced obese rats as a consequence of reduced food intake. Pharmacol Biochem Behav 84:353–359
Bennetzen MF, Nielsen MP, Richelsen B, Pedersen SB (2008) Effects on food intake and blood lipids of cannabinoid receptor 1 antagonist treatment in lean rats. Obesity (Silver Spring) 16:2451–2455
Engeli S, Bohnke J, Feldpausch M et al (2005) Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54:2838–2843
Friedman JE, Dohm GL, Leggett-Frazier N et al (1992) Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J Clin Invest 89:701–705
Rossetti L, Hu M (1993) Skeletal muscle glycogenolysis is more sensitive to insulin than is glucose transport/phosphorylation. Relation to the insulin-mediated inhibition of hepatic glucose production. J Clin Invest 92:2963–2974
Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1:785–789
Rothwell NJ, Stock MJ (1989) Surgical removal of brown fat results in rapid and complete compensation by other depots. Am J Physiol 257:R253–R258
Dulloo AG, Miller DS (1984) Energy balance following sympathetic denervation of brown adipose tissue. Can J Physiol Pharmacol 62:235–240
Cox JE, Lorden JF (1986) Dietary obesity: brown fat denervation fails to alter development or recovery. Am J Physiol 250:R1108–R1116
Yu XX, Lewin DA, Forrest W, Adams SH (2002) Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J 16:155–168
Bartelt A, Bruns OT, Reimer R et al (2011) Brown adipose tissue activity controls triglyceride clearance. Nat Med 17:200–205
Tam J, Vemuri VK, Liu J et al (2010) Peripheral CB1 cannabinoid receptor blockade improves cardiometabolic risk in mouse models of obesity. J Clin Invest 120:2953–2966
Acknowledgements
This work was supported by grants from the National Institutes of Health (DK078283), The American Diabetes Association (research grant 707RA11677), the Cincinnati Mouse Metabolic Phenotyping Center (MMPC DK59630) and Ethicon Endosurgery. Special thanks to S. Lipp and M. Fitzgerald for their outstanding technical assistance.
Contribution statement
All authors substantially contributed to this work by participating in the design and analysis of the experiments, the drafting and revisions of the article and gave final approval of the published version.
Duality of interest
M. Tschöp is a consultant for Roche Pharma and for Ambrx Inc. The remaining authors declare that they have no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Bajzer and M. Olivieri contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 108 kb)
ESM Table 1
Primers for real time Q-PCR (PDF 38 kb)
ESM Figure 1
Body composition and hyperinsulinaemic–euglycaemic clamp studies in DIO mice fed either ad libitum chow [white], 60% ad libitum HFD [black], or HFD-restricted [hatchmarked]. a Fat mass and (b) glucose infusion rate during euglycemic hyperinsulinemic clamp. (n = 5/group) (*p < 0.05 SR vs. PF and VEH) (PDF 74.8 kb)
ESM Figure 2
iBAT temperature measurement with implantable probes during a baseline day, the first day of treatment with 10 mg/kg SR141716 i.p. and the 10th day of treatment. Vehicle-treated [White], and 10 mg/kg SR141716-treated [black] mice. The shaded area indicates the dark period, while the vertical line indicates time of injection. (n = 6), (*p < 0.05) (PDF 78 kb)
ESM Figure 3
Tyrosine hydroxylase protein, a marker of SNS innervation, was measured by IHC (a-f) in iBAT. a No primary antibody (control) on tissue from sham-operated vehicle-treated mice. b Sham-operated vehicle-treated mice. c Denervated vehicle-treated mice. d No primary antibody (control) on tissue from sham-operated SR-treated mice. e Sham-operated SR-treated mice. f Denervated SR-treated mice. a–f (n = 4) (PDF 116 kb)
Rights and permissions
About this article
Cite this article
Bajzer, M., Olivieri, M., Haas, M.K. et al. Cannabinoid receptor 1 (CB1) antagonism enhances glucose utilisation and activates brown adipose tissue in diet-induced obese mice. Diabetologia 54, 3121–3131 (2011). https://doi.org/10.1007/s00125-011-2302-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-011-2302-6