Abstract
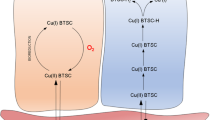
The retention mechanism of the novel imaging/radiotherapeutic agent, Cu-diacetyl-bis(N 4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells was clarified in comparison with that in normal tissuein vitro. With Cu-ATSM and reversed phase HPLC analysis, the reductive metabolism of Cu-ATSM in subcellular fractions obtained from Ehrlich ascites tumor cells was examined. As a reference, mouse brain was used. To determine the contribution of enzymes in the retention mechanisms, and specific inhibitor studies were performed. In subcellular fractions of tumor cells, Cu-ATSM was reduced mainly in the microsome/cytosol fraction rather than in the mitochondria. This finding was completely different from that found in normal brain cells. The reduction process in the microsome/cytosol was heat-sensitive and enhanced by adding exogenous NAD(P)H, an indication of enzymatic reduction of Cu-ATSM in tumor cells. Among the known bioreductive enzymes. NADH-cytochrome b5 reductase and NADPH-cytochrome P450 reductase in microsome played a major role in the reductive retention of Cu-ATSM in tumors. This enzymatic reduction was enhanced by the induction of hypoxia. Radiocopper labeled Cu-ATSM provides useful information for the detection of hypoxia as well as the microsomal bioreductive enzyme expression in tumor.
Similar content being viewed by others
References
Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential.J Nucl Med 1997; 38 (7): 1155–1160.
Fujibayashi Y, Cutler CS, Anderson CJ, McCarthy DW, Jones LA, Sharp T, et al. Comparative studies of Cu-64-ATSM and C-11-acetate in an acute myocardial infarction model:ex vivo imaging of hypoxia in rats.Nucl Med Biol 1999; 26 (1): 117–121.
Lewis JS, McCarthy DW, McCarthy TJ, Fujibayashi Y, Welch MJ. Evaluation of64Cu-ATSMin vitro andin vivo in a hypoxic tumor model.J Nucl Med 1999; 40 (1): 177–183.
Takahashi N, Fujibayashi Y, Yonekura Y, Welch MJ, Waki A, Tsuchida T, et al. Evaluation of62Cu labeled diacetyl-bis(N 4-methylthiosemicarbazone) as a hypoxic tissue tracer in patients with lung cancer.Ann Nucl Med 2000; 14 (5): 323–328.
Welch MJ, Lewis JS, McCarthy DW, Sharp T, Herrero P, McCarthy TJ, et al. Evaluation of copper-60 diacetyl-bis(N 4-methylthiosemicarbazone) (Cu-ATSM), a hypoxia imaging agent in canine models of ischemia.Eur J Nucl Med 1998; 25: 920P.
Takahashi N, Fujibayashi Y, Yonekura Y, Welch MJ, Waki A, Tsuchida T, et al. Copper-62-ATSM as a hypoxic tissue tracer in myocardial ischemia.Ann Nucl Med 2001; 15 (3): 293–296.
Vaupel P, Kelleher DK, Hockel M. Oxygen status of malignant tumors: pathogenesis of hypoxia and significance for tumor therapy.Semin Oncol 2001; 28 (2 Suppl 8): 29–35.
Wardman P. Electron transfer and oxidative stress as key factors in the design of drugs selectively active in hypoxia.Curr Med Chem 2001; 8 (7): 739–761.
Connett JM, Anderson CJ, Guo LW, Schwarz SW, Zinn KR, Rogers BE, et al. Radioimmunotherapy with a64Cu-labeled monoclonal antibody: a comparison with67Cu.Proc Natl Acad Sci USA 1996; 93 (13): 6814–6818.
Lewis J, Laforest R, Buettner T, Song S, Fujbaayashi Y, Connett J, et al. Copper-64-diacetyl-bis(N 4-methylthiosemicarbazone): An agent for radiotherapy.Proc Natl Acad Sci USA 2001; 98 (3): 1206–1211.
Petering DH.Carcinostatic Copper Complexes, in Metal ions in biological systems, Siegel H (ed), New York; Marcel Dekker, 1980: 197–229.
Fujibayashi Y, Wada K, Taniuchi H, Yonekura Y, Konishi J, Yokoyama A. Mitochondria-selective reduction of62Cu-pyruvaldehyde bis(N 4-methylthiosemicarbazone) (62Cu-PTSM) in the murine brain; a novel radiopharmaceutical for brain positron emission tomography (PET) imaging.Biol Pharm Bull 1993; 16 (2): 146–149.
Shibuya K, Fujibayashi Y, Yoshimi E, Sasai K, Hiraoka M, Welch MJ. Cytosolic/microsomal redox pathway: a reductive retention mechanism of a PET-oncology tracer, Cu-pyruvaldehyde-bis(N 4-methylthiosemicarbazone) (Cu-PTSM).Ann Nucl Med 1999; 13 (5): 287–292.
Gingas BA, Suprunchuk T, Bayley CH. The preparation of some thiosemicarbazones and their copper complexes. Part III.Can J Chem 1962; 40: 1053–1059.
Fujibayashi Y, Taniuchi H, Wada K, Yonekura Y, Konishi J, Yokoyama A. Differential mechanism of retention of Cu-pyruvaldehyde-bis(N 4 (N 4-methylthiosemicarbazone) (Cu-PTSM) by brain and tumor: a novel radiopharmaceutical for positron emission tomography imaging.Ann Nucl Med 1995; 9 (1): 1–5.
Minkel DT, Saryan LA, Petering DH. Structure-function correlations in the reaction of bis (thiosemicarbazonato) copper (II) complexes with Ehrlich ascites tumor cells.Cancer Res 1978; 38: 124–129.
Voss DO, Camplleo AP, Bacila M. The respiratory chain and the oxidative phosphorylation of rat brain mitochondria.Biochem Biophys Res Commun 1961; 4: 48–51.
Taniuchi H, Fujibayashi Y, Yonekura Y, Konishi J, Yokoyama A. Hyperfixation of copper-62-PTSM in rat brain after transient global ischemia.J Nucl Med 1997; 38 (7): 1130–1134.
Rauth AM, Marshall RS, Kuehl BL. Cellular approaches to bioreductive drug mechanisms.Cancer Metastasis Rev 1993; 12: 153–164.
Siegel D, Gibson NW, Preusch PC, Ross D. Metabolism of mitomycin C by DT-diaphorase: role in mitomycin C-induced DNA damage and cytotoxicity in human colon carcinoma cells.Cancer Res 1990; 50 (23): 7483–7489.
Joseph P, Xu Y, Jaiswal AK. Non-enzymatic and enzymatic activation of mitomycin C: identification of a unique cytosolic activity.Int J Cancer 1996; 65 (2): 263–271.
Ernster I. DT diaphorase.Methods Enzymol 1967; 10: 309–317.
Walton MI, Workman P. Enzymology of the reductive bioactivation of SR 4233. A novel benzotriazine di-N-oxide hypoxic cell cytotoxin.Biochem Pharmacol 1990; 39 (11): 1735–1742.
Barham HM, Stratford IJ. Enzymology of the reduction of the novel fused pyrazine mono-n-oxide bioreductive drug, RB90740 roles for P450 reductase and cytochrome b5 reductase.Biochem Pharmacol 1996; 51 (6): 829–837.
Stuehr DJ, Fasehun OA, Kwon NS, Gross SS, Gonzalez JA, Levi R, et al. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs.Faseb J 1991; 5 (1): 98–103.
Lostanlen D, Vieira de Barros A, Leroux A, Kaplan JC. Soluble NADH-cytochrome b5 reductase from rabbit liver cytosol: partial purification and characterization.Biochem Biophys Acta 1978; 526: 42–51.
Karplus PA, Daniels MJ, Herriott JR. Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family.Science 1991; 251 (4989): 60–66.
Lee E, Kariya K. Propylthiouracil, a selective inhibitor of NADH-cytochrome b5 reductase.FEBS Lett 1986; 209 (1): 49–51.
Coats EA, Milstein SR, Holbein G, McDonald J, Reed R, Petering HG. Comparative analysis of the cytotoxicity of substituted (phenylglyoxal bis(4-methyl-3-thiosemicarbazone)) copper(II) chelates.J Med Chem 1976; 19: 131–135.
Minkel DT, Petering DH. Initial reaction of 3-ethoxy-2-oxobutyraldehyde bis(thiosemicarbazonato) copper(II) with Ehrlich ascites tumor cells.Cancer Res 1978; 38: 117–123.
Taniuchi H, Fujibayashi Y, Okazawa H, Yonekura Y, Konishi J, Yokoyama A. Cu-pyruvaldehyde-bis(N 4-methylthiosemicarbazone) (Cu-PTSM), a metal complex with selective NADH-dependent reduction by complex I in brain mitochondria: a potential radiopharmaceutical for mitochondria-functional imaging with positron emission tomography (PET).Biol Pharm Bull 1995; 18 (8): 1126–1129.
Stratford IJ, Workman P. Bioreductive drugs into the next millennium.Anticancer Drug Des 1998; 13 (6): 519–528.
Jaffar M, Naylor MA, Robertson N, Stratford IJ. Targeting hypoxia with a new generation of indolequinones.Anticancer Drug Des 1998; 13 (6): 593–609.
Patterson AV, Barham HM, Chinje EC, Adams GE, Harris AL, Stratford IJ. Importance of P450 reductase activity in determining sensitivity of breast tumour cells to the bioreductive drug, tirapazamine (SR 4233).Br J Cancer 1995; 72 (5): 1144–1150.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obata, A., Yoshimi, E., Waki, A. et al. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent Cu-diacetyl-bis(N 4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann Nucl Med 15, 499–504 (2001). https://doi.org/10.1007/BF02988502
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02988502