Abstract
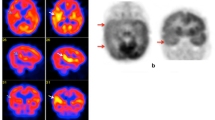
Iodine-123 iomazenil (IMZ) has excellent characteristics for the quantification of central benzodiazepine receptor (BZR) binding with single-photon emission tomography (SPET). In order to evaluate the clinical value of IMZ SPET for presurgical identification of epileptic foci in patients with medically intractable seizures, we measured the binding potential (BP) of BZR using two IMZ SPET scans and compared the results with brain perfusion SPET and fluorine-18 fluorodeoxyglucose (FDG) positron emission tomography (PET). A total of ten patients with intractable partial epilepsy were examined by electroencephalography, magnetic resonance imaging, FDG PET, brain perfusion SPET and IMZ SPET. After neuroimaging examinations, five patients underwent selective surgery, and all of them have since been free of seizures. Two SPET scans were performed at 15 min (early) and 3 h (late) after intravenous injection of123I-IMZ (167 MBq). Parametric images of the ligand transport (K 1) and binding potential (BP) were calculated by the table look-up method, which is based on a three-compartment two-parameter model, using the standard arterial input function obtained by averaging of six normal volunteers' input functions. BP images delineated the epileptic foci more precisely than either FDG PET or ictal perfusion SPET. FDG PET showed widespread reduction, including the area surrounding the focus, and ictal increase in the cerebral blood flow was seen in possibly activated areas spread from the focus. In four epilepsy cases which originated from the mesial temporal lobe without lateral temporal abnormality, there was no significant decrease in the BP images in the lateral temporal structures, which showed decreased uptake of FDG. It is concluded that parametric images of BP with IMZ are valuable for precise presurgical localization of epileptic foci.
Similar content being viewed by others
References
Spencer SS. The relative contributions of MRI, SPECT, and PET imaging in epilepsy.Epilepsia 1994; 35: S72-S89.
Jibiki I, Yamaguchi N. Epilepsy and SPECT.Neurosci Biobehav Rev 1994; 18: 281–290.
Nagata T, Tanaka F, Yonekura Y, et al. Limited value of interictal brain perfusion SPECT for detection of epileptic foci: high resolution SPECT studies in comparison with FDG-PET.Ann Nucl Med 1995; 9: 59–63.
Engel J, Henry TR, Risinger MW, et al. Presurgical evaluation for epilepsy: relative contribution of chronic depth electrode recording versus FDG-PET and scalp-sphenoidal ictal EEG.Neurology 1990; 40: 1670–1677.
Sackellares JC, Siegel GJ, Abou-Khalil BW, et al. Differences between lateral and mesial temporal metabolism interictally in epilepsy of mesial temporal origin.Neurology 1990; 40: 1420–1426.
Hajek M, Antonini A, Leenders KL, et al. Mesiobasal versus lateral temporal lobe epilepsy: metabolic differences in the temporal lobe shown by interictal18F-FDG positron emission tomography.Neurology 1993; 43: 79–86.
Rowe CC, Berkovic SF, Austin MC, et al. Patterns of postictal cerebral blood flow in temporal lobe epilepsy: qualitative and quantitative analysis.Neurology 1991; 41: 1096–1103.
Duncan R, Patterson J, Roberts R, et al. Ictal/postictal SPECT in the presurgical localization of complex partial seizures.J Neurol Neurosurg Psychiatry 1993; 56: 141–148.
Newton MR, Berkovic SF, Austin MC, et al. Postictal switch in blood flow distribution and temporal lobe seizures.J Neurol Neurosurg Psychiatry 1992; 55: 819–894.
Newton MR, Berkovic SF, Austin MC, et al. Ictal postictal and interictal single-photon emission tomography in the lateralization of temporal lobe epilepsy.J Nucl Med 1994; 21: 1067–1071.
Savic I, Persson A, Roland O, et al. In-vivo demonstration of reduced benzodiazepine-receptor binding in human epileptic foci.Lancet 1988; 863–866.
Henry TR, Frey KA, Sackellares JC, et al. In vivo cerebral metabolism and central benzodiazepine-receptor binding in temporal lobe epilepsy.Neurology 1993; 43: 1998–2006.
Savic I, Ingvar M, Stone-Elander S. Comparison of [11C]flumazenil and [18F]FDG as PET markers of epileptic foci.J Neurol Neurosurg Psychiatry 1993; 56: 615–621.
van Huffelen AC, van Isselt JW, van Veelen CWM, et al. Identification of the side of the epileptic focus with123I-iomazenil SPECT. A comparison with18FDG-PET and ictal EEG findings in patients with medically intractable complex partial seizures.Acta Neurochir Suppl (Wien) 1990; 50: 95–99.
Bartenstein P, Ludolph A, Schober O, et al. Benzodiazepine receptors and cerebral blood flow in partial epilepsy.Eur J Nucl Med 1991; 18: 111–118.
Schubiger PA, Hasler PH, Beer-Wohlfahrt H, et al. Evaluation of a multicentre study with iomazenil — a benzodiazepine ligand.Nucl Med Commun 1991; 12: 569–582.
Ferstl FJ, Cordes M, Cordes I, et al. 123-I-Iomazenil-SPECT in patients with focal epilepsies — a comparative study with99mTc-HMPAO-SPECT, CT and MR.Adv Exp Med Biol 1991; 287: 405–412.
Johnson EW, de Lanerolle NC, Kim JH, et al. “Central” and “peripheral” benzodiazepine receptors: opposite changes in human epileptogenic tissue.Neurology 1992; 42: 811–815.
Cordes M, Henkes H, Ferstl F, et al. Evaluation of focal epilepsy: a SPECT scanning comparison of 123-I-Iomazenil versus HM-PAO.Am J Neuroradiol 1992; 13: 249–253.
Duncan S, Gillen GJ, Brodie MJ. Lack of effect of concomitant clobazam on interictal123I-iomazenil SPECT.Epilepsy Res 1993; 15: 61–66.
Onishi Y, Yonekura Y, Muaki T, et al. Simple quantification of benzodiazepine receptor binding and ligand transport using iodine-123-iomazenil and two SPECT scans.J Nucl Med 1995; 36: 1201–1210.
Onishi Y, Yonekura Y, Nishizawa S, et al. Noninvasive method of iodine-123-iomazenil SPECT.J Nucl Med 1996; 37: 374–378.
Jibiki I, Yamaguchi N, Matsuda H, et al. Paradoxical enhancement of hypoperfusion single photon emission CT images in epileptic focus with bemegride activation.Eur Neurol 1992; 32: 146–150.
Beer HF, Blauenstein PA, Hasler PH, et al. In vitro and in vivo evaluation of iodine-123-Ro 16-0154: a new imaging agent for SPECT investigations of benzodiazepine receptors.J Nucl Med 1990; 31: 1007–1014.
Woods SW, Seibyl JP, Goddard AW, et al. Dynamics SPECT imaging of the benzodiazepine receptor in healthy human subjects with [123I]Ro 16-0154.Psychiatry Res: Neuroimaging 1992; 45: 67–77.
Hoell K, Deisenhammer E, Dauth J, et al. Imaging benzodiazepine receptors in the human brain by single photon emission computed tomography (SPECT).Nucl Med Biol 1989; 16: 759–763.
Lassen NA, Blasberg RG. Technetium-99m-d,l-HMPAO, the development of a new class of99mTc-labeled tracers: an overview.J Cereb Blood Flow Metab 1988; 8: S1-S3.
Yonekura Y, Ishizu K, Okazawa H, et al. Simplified quantification of regional cerebral blood flow with99mTc-ECD SPECT and continuous arterial sampling.Ann Nucl Med 1996; 10: 177–183.
Tsuchida T, Nishizawa S, Yonekura Y, et al. SPECT images of technetium-99m-ethyl cysteinate dimer in cerebrovascular disease: comparison with other cerebral perfusion tracers and PET.J Nucl Med 1994; 35: 27–31.
Yonekura Y, Nishizawa S, Mukai T, et al. Functional mapping of flow and back-diffusion rate ofN-isopropyl-p-iodoamphetamine in human brain.J Nucl Med 1993; 34: 839–844.
Spencer SS, Williamson PD, Bridgers SL, et al. Reliability and accuracy of localization by scalp ictal EEG.Neurology 1985; 35: 1567–1575.
Sammaritano M, de Lotbiniere A, Andermann F, et al. False lateralization by surface EEG of seizure onset in patients with temporal lobe epilepsy and gross focal cerebral lesions.Ann Neurol 1987; 21: 361–369.
Engel J, Brown WJ, Kuhl DE, et al. Pathological findings underlying focal temporal lobe hypometabolism in partial epilepsy.Ann Neurol 1982; 12: 518–528.
Morrell F. Secondary epileptogenesis in man.Arch Neurol 1985; 42: 318–335.
Spencer SS, Williamson PD, Spencer DD, et al. Human hippocampal seizure spread studied by depth and subdural recording: the hippocampal commissure.Epilepsia 1987; 28: 479–489.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tanaka, F., Yonekura, Y., Ikeda, A. et al. Presurgical identification of epileptic foci with iodine-123 iomazenil SPET: Comparison with brain perfusion SPET and FDG PET. Eur J Nucl Med 24, 27–34 (1997). https://doi.org/10.1007/BF01728305
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01728305