Summary
Many steps in melanoma metastasis involve cell-cell or cell-matrix adhesive interactions. The surface molecules which mediate these processes therefore play an important role in regulating melanoma dissemination and their level of expression may alter during the course of tumor progression. Human melanocyte strains and melanoma cell lines have been characterised with regard to levels of cell surface receptors of the integrin family. Increased amounts of at least two integrins, VLA-4 (α4β1) and VnR (αVβ3), appeared to correlate with progression in this tumor, type. A novel VnR composed of an αVβ1 association has been observed in one melanoma cell line and there is the possibility that heterogeneity of integrin composition could affect biological behavior of these tumors.
CD44, a cell surface glycoprotein which functions as the major receptor for hyaluronate, is another molecule whose expression increases in transformed cells of the melanocytic lineage. Iterative sorting on the FACS for stable variants, of both human and murine melanomas, expressing low and high levels of CD44 established that lack of expression of this molecule correlated with impaired ability to form pulmonary tumor nodules subsequent to i.v. injection into appropriate recipient mice. These findings illustrate that an understanding of the regulation of melanoma adhesion receptors could provide insights into the process of tumor spread.
Similar content being viewed by others
References
Mastrangelo MJ, Baker AR, Katz HR: Cutaneous Melanoma. DeVita VT, Hellman S, Rosenberg SA, (es), In: Cancer: Principles and Practice of Oncology. 2nd Edition. pp. 1371–1422, J. B. Lippincott, Philadelphia, 1985
Hart IR, Goode NT, Wilson RE: Molecular aspects of the metastatic cascade. Biochim Biophys Acta 989: 65–84, 1989
Liotta LA: Tumor invasion and metastasis-role of the extracellular matrix. Cancer Res 46: 1–7, 1986
LeDouarin N: The Neural Crest. Cambridge University Press, London, 1982
Weston JA: The migration and differentiation of neural crest cells. Adv Morphog 8: 41–114, 1970
Brauer PR, Bolender DL, Markwald RR: The distribution and spatial organization of the extracellular matrix encountered by mesencephalic neural crest cells. Anat Rec 211: 57–68, 1985
Tosney KW: The segregation and early migration of cranial neural crest cells in the avian embryo. Dev Biol 89: 13–24, 1982
Passaniti A, Hart GW: Metastasis-associated murine melanoma cell surface galactosyltransferase: Characterization of enzyme activity and identification of the major surface substrates. Cancer Res 50: 7261–7271, 1990
Runyan RB, Maxwell GD, Shur BD: Evidence for a novel enzymatic mechanism of neural crest cell migration on extracellular glycoconjugate matrices. J Cell Biol 102: 432–441, 1986
Nowell PC: The clonal evolution of tumor cell populations. Science 194: 23–28, 1976
Clark W, Elder D, Guerry D, Epstein M, Greene M, VanHorn M: A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol 15: 1147–1165, 1984
Clark W, Elder D, VanHorn M: The biologic forms of malignant melanoma. Hum Pathol 17: 443–452, 1986
Brooks G, Birch M, Hart IR: Effects of biologically active tumor-promoting and non-promoting phorbol esters on in vitro growth of melanocytic cells. Pigment Cell Res 3: 98–100, 1990
Eisinger M, Marko O: Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci USA 79: 2018–2022, 1982
Eisinger M, Marko O, Weinstein IB: Stimulation of growth of human melanocytes by tumor promoters. Carcinogenesis 4: 779–781, 1983
Holzmann B, Brocker EB, Lehmann JM, Ruiter DJ, Sorg C, Reithmuller G, Johnson JP: Tumor progression in human malignant melanoma: five stages defined by their antigenic phenotypes. Int J Cancer 39: 466–471, 1987
Holzmann B, Lehmann JM, Ziegler-Heitbrock L, Funke I, Riethmuller G, Johnson JP: Glycoprotein P3.58, associated with tumor progression in malignant melanoma, is a novel leukocyte activation antigen. Int J Cancer 41: 542–547, 1988
Johnson JP, Stade BG, Holzmann B, Schwable W, Riethmuller G: De novo expression of intercellular adhesion molecule 1 in melanoma correlates with increased risk of metastasis. Proc Natl Acad Sci USA 86: 641–644, 1989
Lehmann JM, Riethmuller G, Johnson JP: MUC 18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci USA 86: 9891–9895, 1989
Cheresh DA, Pierschbacher MD, Herzig MA, Mujoo K: Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J Cell Biol 102: 688–696, 1986
Iliopoulos D, Ernst C, Steplewski Z, Jambrosic JA, Rodeck U, Herlyn M, Clark WH, Koprowski H, Herlyn D: Inhibition of metastases of a human melanoma xenograft by monoclonal antibody to the GD2/GD3 gangliosides. J Natl Cancer Inst 81: 440–444, 1989
Juliano RL: Membrane receptors for extracellular matrix macromolecules: relationship to cell adhesion and tumor metastasis. Biochim Biophys Acta 907: 261–278, 1987
McCarthy JB, Basara ML, Palm SL, Sas DF, Furcht LT: The role of cell adhesion proteins-laminin and fibronectin-in the movement of malignant and metastatic cells. Cancer Metastasis Rev 4: 125–152, 1985
Nicolson GL: Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Current Opinion in Cell Biol 1: 1009–1019, 1989
Hynes RO: Integrins: A family of cell surface receptors. Cell 48: 549–554, 1987
Hogg N: The leukocyte integrins. Immunology Today 10: 111–114, 1989
Ruoslahti E, Pierschbacher MD: New perspectives in cell adhesion; RGD and Integrins. Science 238: 491–497, 1987
Hemler ME: VLA proteins in the integrin family: structures, functions and their role on leukocytes. Ann Rev Immunol 8: 365–400, 1990
Phillips DR, Charo IF, Parise LV, Fitzgerald LA: The platelet membrane glycoprotein IIb-IIIa complex. Blood 71: 831–843, 1988
Cheresh DA, Smith JW, Cooper HM, Quaranta V: A novel vitronectin receptor integrin (αVβV) is responsible for distinct adhesive properties of carcinoma cells. Cell 57: 59–69, 1989
Freed E, Gailit J, van derGreer P, Ruoslahti E, Hunter T: A novel integrin β subunit is associated with the vitronectin receptor α subunit (αV) in a human osteosarcoma line and is a substrate for protein kinase C. EMBO J 8: 2955–2965, 1989
Holzmann B, McIntyre BW, Weissman IL: Identification of a murine Peyer's patch-specific lymphocyte homing receptor as integrin molecule with an α chain homologous to human VLA-4α. Cell 56: 37–46, 1989
Kajiji S, Tamura RN, Quaranta V: A novel integrin (αEβ4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. The EMBO J 8: 673–680, 1989
Krissansen GW, Elliot MJ, Lucas CM, Stornski FC, Berndt MC, Cheresh DA, Lopez AF, Burns GF: Identification of a novel integrin β subunit expressed on cultured monocytes (macrophages): evidence that one α subunit can associate with multiple β subunits. J Biol Chem 265: 823–830, 1990
Sheppard D, Rozzo C, Starr L, Quaranta V, Erle DJ, Pytela R: Complete amino acid sequence of a novel integrin β subunit (β6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem 265: 11502–11507, 1990
Bodary SC, McLean JW: The integrin β1 subunit associates with the vitronectin receptor αV subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem 265: 5938–5941, 1990
Vogel BE, Tarone G, Giancotti FG, Gailit J, Ruoslahti E: A novel fibronectin receptor with an unexpected subunit composition (αVβ1). J Biol Chem 265: 5934–5937, 1990
Takada Y, Wayner EA, Carter WG, Hemler ME: Extracellular matrix receptors, ECMRII and ECMRI, for collagen and fibronectin, correspond to VLA-2 and VLA-3 in the VLA family of heterodimers. J Cell Biochem 37: 385–393, 1988
Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ: The function of extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol 107: 1881–1891, 1988
Elices MJ, Hemler ME: The human VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci USA 86: 9906–9910, 1989
Kirchoffer D, Languino LR, Ruoslahti E, Pierschbacher MD: 126–1 integrins from different cell types show different binding specificities. J Biol Chem 265: 615–618, 1990
Conforti G, Zanetti A, Pasquali-Ronchetti I, Quaglino D, Neyroz P, Dejana E: Modulation of vitronectin receptor by membrane lipid composition. J Biol Chem 265: 4011–4019, 1990
Gehlsen KR, Argraves WS, Pierschbacher MD, Ruoslahti E: Inhibition of in vitro tumour cell invasion by Arg-Gly-Asp containing synthetic peptides. J Cell Biol 106: 925–930, 1988
Humphries MJ, Olden K, Yamada KM: A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science 233: 467–470, 1986
Saiki I, Murata J, Iida J, Nishi N, Sugimura K, Azuma I: The inhibition of murine lung metastasis by synthetic polypeptides [poly(arg-gly-asp) and poly(tyr-ile-gly-ser-arg)] with a core sequence of cell adhesion molecules. Br J Cancer 59: 194–197, 1989
Gehlsen KR, Dillner L, Engvall E, Ruoslahti E: The human laminin receptor is a member of the integrin family of cell adhesion receptors. Science 241: 1228–1229, 1988
Terranova VP, Rao CN, Kalebic T, Margulies IM, Liotta LA: Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci USA 80: 444–448, 1983
Graf J, Iwamoto Y, Sasaki M, Martin GR, Kleinman HK, Robey FA, Yamada Y: Identification of an amino acid sequence in laminin mediating cell attachment, chemotaxis and receptor binding. Cell 48: 989–996, 1987
Iwamoto Y, Robey FA, Graf J, Sasaki M, Kleinman HK, Yamada Y, Martin GR: YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science 238: 1132–1134, 1987
Kramer RH, Marks N: Identification of integrin collagen receptors on human melanoma cells. J Biol Chem 264: 4684–4688, 1989
Kramer RH, McDonald KA, Crowley E, Ramos DM, Damsky CH: Melanoma cell adhesion to basement membrane mediated by integrin-related complexes. Cancer Res 49: 393–402, 1989
Ramos DM, Berston ED, Kramer RH: Analysis of integrin receptors for laminin and type IV collagen on metastatic B16 melanoma cells. Cancer Res 50: 728–734, 1990
Plantefaber LC, Hynes RO. Changes in integrin receptors on oncogenically transformed cells. Cell 56: 281–290, 1989
Giancotti FG, Ruoslahti E: Elevated levels of the α5β1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell 60: 849–859, 1990
Kramer RH, McDonald KA, Vu MP: Human melanoma cells express a novel integrin receptor for laminin. J Biol Chem 264: 15642–15649, 1989
Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, Sonnenberg A, Damsky CH: The α1/β1 and α6/β1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol 110: 2175–2184, 1990
Newgreen DF: Spreading of explants of embryonic chick mesenchymes and epithelia on fibronectin and laminin. Cell Tissue Res 236: 265–277, 1984
Albelda SM, Mette SA, Elder DE, Stewart R, Damjanovich L, Herlyn M, Buck CA: Integrin distribution in malignant melanoma: Association of the β3 subunit with tumor progression. Cancer Res 50: 6757–6764, 1990
Hemler ME, Huang C, Takada Y, Schwarz L, Strominger JL, Clabby ML: Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem 262: 11478–11485, 1987
Holzmann B, Weissman IL: Peyer's patch-specific lymphocyte homing receptors consist of a VLA-4-like α-chain associated with either of two integrin β-chains, one of which is novel. The EMBO J 8: 1735–1741, 1989
Takada Y, Elices MJ, Crouse C, Hemler ME: The primary structure of the α4 subunit of VLA-4: homology to other subunits and a possible cell-cell adhesion function. The EMBO J 8: 1361–1368, 1989
Mould AP, Wheldon LA, Komoriya A, Wayner EA, Yamada KM, Humphries MJ: Affinity chromatographic isolation of the melanoma adhesion receptor for the IIICS region of fibronectin and its identification as the integrin α4β1. J Biol Chem 265: 4020–4024, 1990
Jalkanen S, Reichert RA, Gallatin WM, Bargatze RF, Weissman IL, Butcher EC: Homing receptors and the control of lymphocyte migration. Immunol Rev 91: 39–60, 1986
Sher BT, Bargatze R, Holzmann B, Gallatin WM, Mathews D, Wu N, Picker L, Butcher EC, Weissman IL: Homing receptors and metastasis. Adv Cancer Res 51: 361–390, 1988
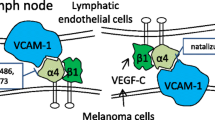
Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S, Chi-Rosso G, Lobb R: Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell 59: 1203–1211, 1989
Rice GE, Munro JM, Bevilacqua MP, Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. J Exp Med 171: 1369–1374, 1990
Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, Lobb R: VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell 60: 577–584, 1990
Rice GE, Bevilacqua MP: An inducible endothelial cell surface glycoprotein mediates melanoma adhesion. Science 246: 1303–1306, 1989
Rice GE, Gimbrone MA, Bevilacqua MP: Tumor cell-endothelial interactions: increased adhesion of human melanoma cells to activated vascular endothelium. Am J Pathol 133: 204–210, 1988
Wayner EA, Carter WG, Piotrowicz RS, Kunicki TJ: The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: Preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol 107: 1881–1891, 1988
Cheresh DA, Smith JW, Cooper HM, Quaranta V: A novel vitronectin receptor integrin (αVβX) is responsible for distinct adhesive properties of carcinoma cells. Cell 57: 59–69, 1989
Sonnenberg A, Modderman PW, Hogervorst F: Laminin receptor on platelets is the integrin VLA-6. Nature 360: 487–489, 1988
Hemler ME, Crouse C, Sonnenberg A: Association of the VLAα6 subunit with a novel protein: A possible alternative to the common VLAβ1 subunit on certain cell lines. J Biol Chem 264: 6529–6535, 1989
Kennel SJ, Foote LJ, Falcioni R, Sonnenberg A, Stringer CD, Crouse C, Hemler ME: Analysis of the tumor-associated antigen TSP-180. Identity with α-β4 in the integrin superfamily. J Biol Chem 264: 15515–15521, 1989
Constantini RM, Falcioni R, Battista P, Zupi G, Kennel SJ, Colasante A, Venturo I, Curcio CG, Sacchi A: Integrin (α6/β4) expression in human lung cancer as monitored by specific monoclonal antibodies. Cancer Res 50: 6107–6112, 1990
Falcioni R, Kennel SJ, Giacomini P, Zupi G, Sacchi A: Expression of tumor antigen correlated with metastatic potential of Lewis lung carcinoma and B16 melanoma clones in mice. Cancer Res 46: 5772–5778, 1986
Cheresh DA, Spiro RC: Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen and von Willebrand factor. J Biol Chem 262: 17703–17711, 1987
Lawler J, Weinstein R, Hynes RO: Cell attachment to thrombospondin: the role of Arg-Gly-Asp, and integrin receptors. J Cell Biol 107: 2351–2361, 1988
Oldberg A, Franzen A, Heinegard D, Pierschbacher M, Ruoslahti E: Identification of a bone sialoprotein receptor in osteosarcoma cells. J Biol Chem 263: 19433–19436, 1988
Kramer RH, Cheng Y-F, Clyman R: Human microvascular endothelial cells use β1 and β3 integrin receptor complexes to attach to laminin. J Cell Biol 111: 1233–1243, 1990
McGregor BC, McGregor JL, Weiss LM, Wood GS, Hu C-H, Boukerche H, Warnke RA: Presence of cytoadhesins (IIb-IIIa-like glycoproteins) on human metastatic melanomas but not on benign melanocytes. J Am Clin Pathol 92: 495–499, 1989
Boukerche H, Berthier-Vergnes O, Bailly M, Dore JF, Leung LLK, McGregor JL: A monoclonal antibody (LYP18) directed against the blood platelet glycoprotein IIb/IIIa complex inhibits melanoma growth in vivo. Blood 74: 909–912, 1989
Ruoslahti E, Giancotti FG: Integrins and tumour cell dissemination. Cancer Cells 1: 119–126, 1989
Nip J, Brodt P: Evidence that the vitronectin receptor plays a role in lymphatic dissemination of human melanoma. Clin Expl Metastasis 8: (Supplement 1): 41A, 1990
Schreiner CL, Bauer JS, Danilov YS, Hussein S, Sczekan MM, Juliano RL. Isolation and characterization of Chinese Hamster Ovary Cell variants deficient in the expression of fibronectin receptor. J Cell Biol 109: 3157–3167, 1989
Shimizu Y, VanSeventer GA, Horgan KJ, Shaw S: Regulated expression and binding of three VLA (β1) integrin receptors on T cells. Nature 345: 250–253, 1990
Vandenbark GR, Kuhn LJ, Niedel JE: Possible mechanisms of phorbol diester-induced maturation of human promyelocytic leukemia cells: activation of protein kinase C. J Clin Invest 73: 448–457, 1984
Horvath AR, Elmore MA, Kellie S: Differential tyrosine-specific phosphorylation of integrin in Rous sarcoma virus transformed cells with differing transformed phenotypes. Oncogene 5: 1349–1357, 1990
Gopalakrishna R, Barsky SH: Tumor promoter-induced membrane-bound protein kinase C regulates hematogenous metastasis. Proc Natl Acad Sci USA 85: 612–616, 1988
Springer TA: Adhesion receptors of the immune system. Nature 346: 425–434, 1990
Gowans JK, Knight EJ: The route of recirculation of lymphocytes in the rat. Proc R Soc (Lond) B 159: 257–282, 1964
Holzmann B, McIntyre BW, Weissman IL: Identification of a murine Peyer's patch-specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA-4α Cell 56: 3746, 1989
Dalchau R, Kirkley J, Fabre JW: Monoclonal antibody to a human brain-granulocyte-T lymphocyte antigen probably homologous to the W3/13 antigen of the rat. Eur J Immunol 10: 745–749, 1980
Flanagan BF, Dalchau R, Allen AK, Daar AS, Fabre JW: Chemical composition and tissue distribution of the human CDW44 glycoprotein. Immunology 67: 167–175, 1989
Goldstein LA, Zhou DFH, Picker LJ, Minty CN, Bargatze RF, Ding JF, Butcher EC: A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell 56: 1063–1072, 1989
Stamenkovic I, Amiot M, Pesando JM, Seed B: A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell 56: 1057–1062, 1989
St.John T, Meyer J, Idzerda R, Gallatin WM: Expression of CD44 confers a new adhesion phenotype on transfected cells. Cell 60: 45–52, 1990
Jalkanen S, Bargatze RF, Herron LR, Butcher EC: A lymphoid cell surface glycoprotein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol 16: 1195–1202, 1986
Isacke CM, Sauvage CA, Hyman R, Lesley J, Schulte R, Trowbridge IS: Identification and characterisation of the human Pgp-1 glycoprotein. Immunogenetics 23: 326–332, 1986
Carter WG, Wayner EA: Characterisation of the class 111 collagen receptor, a phosphorylated transmembrane glycoprotein expressed in nucleated human cells. J Biol Chem 263: 4193–4201, 1988
Picker LJ, de losToyos J, Telen MJ, Haynes BF, Butcher EC: Monoclonal antibodies against the CD44 [In (Lu)-related p 80], and Pgp-1 antigens in man recognize the Hermes class of lymphocyte homing receptors. J Immunol 142: 2046–2051, 1989
Nottenburg C, Rees G, St.John T: Isolation of mouse CD44 cDNA: Structural features are distinct from the primate cDNA. Proc Natl Acad Sci USA 86: 8521–8525, 1989
Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B: CD44 is the principal cell surface receptor for hyaluronate. Cell 61: 1303–1313, 1990
Miyake K, Underhill CB, Lesley J, Kincade PW: Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med 172: 69–75, 1990
Laurent TC, Fraser RE: Properties and turnover of hyaluronan. In ‘Functions of Proteoglycans’: Ciba Foundation Symposium 124. pp 9–29, Chichester, 1986
Ruoslahti E: Structure and biology of proteoglycans. Ann Rev Cell Biol 4: 229–255, 1988
Toole BP, Biswas C, Gross J: Hyaluronate and invasiveness of the rabbit V2 carcinoma. Proc Natl Acad Sci USA 76: 6299–6305, 1979
Nemec RE, Toole BP, Knudson W: The cell surface hyaluronate binding sites of invasive human bladder carcinoma cells. Biochem Biophys Res Commun 149: 249–257, 1987
Green SJ, Tarone G, Underhill CB: Distribution of hyaluronate and hyaluronate receptors in the adult lung. J Cell Sci 89: 145–156, 1988
Pessac B, Defendi V: Cell aggregation: Role of acid mucopolysaccharide. Science 175: 898–900, 1972
Underhill CB, Dorfman A: The role of hyaluronic acid in intercellular adhesion of cultured mouse cells. Expl Cell Res 117: 155–164, 1978
Underhill CB, Toole BP: Binding of hyaluronate to the surface of cultured cells. J Cell Biol 82: 475–481, 1979
Fidler IJ: The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur J Cancer 9: 223–227, 1973
Liotta LA, Mandler R, Murano G, Katz DA, Gordon RK, Ching PK, Schiffmann E: Tumor cell autocrine motility factor. Proc Natl Acad Sci USA 83: 3302–3306, 1986
McCarthy JB, Hagen ST, Furcht LT: Human fibronectin contains distinct adhesion- and motility-promoting domains for metastatic melanoma cells. J Cell Biol 102: 179–188, 1986
Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W: Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol 111: 2097–2108, 1990
Lacy BE, Underhill CB: The hyaluronate receptor is associated with actin filaments. J Cell Biol 105: 1395–1404, 1987
Hughes EN, Mengod M, August JT: Murine cell surface glycoproteins. Characterization of a major component of 80,000 daltons as a polymorphic differentiation antigen of mesenchymal cells. J Biol Chem 256: 7023–7027, 1981
Moczar M, Poupon M-F, Moczar E: Hyaluronate-binding proteins in weakly and highly metastatic variants of rat rhabdomyosarcoma cells. Clin Exptl Met 8: 129–140, 1990
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hart, I.R., Birch, M. & Marshall, J.F. Cell adhesion receptor expression during melanoma progression and metastasis. Cancer Metast Rev 10, 115–128 (1991). https://doi.org/10.1007/BF00049409
Issue Date:
DOI: https://doi.org/10.1007/BF00049409