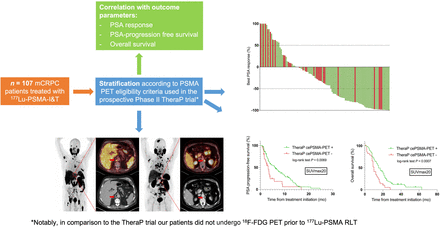
Visual Abstract
Abstract
Prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) has shown encouraging results for treatment of metastatic castration-resistant prostate cancer (mCRPC) in the prospective, multicenter, randomized phase II TheraP study. The inclusion criteria for that study comprised a pretherapeutic 68Ga-PSMA-11 PET scan showing sufficient tumor uptake using a predefined threshold and the absence of 18F-FDG–positive, PSMA ligand–negative tumor lesions. However, the prognostic value of these PET-based inclusion criteria remains unclear. Therefore, we evaluated the outcome of mCRPC patients treated with PSMA RLT using TheraP as well as other TheraP-based PET inclusion criteria. Methods: First, patients were dichotomized into 2 groups whose PSMA PET scans did (TheraP contrast-enhanced PSMA [cePSMA] PET–positive) or did not (TheraP cePSMA PET–negative) fulfill the inclusion criteria of TheraP. Notably, unlike in TheraP, 18F-FDG PET was not performed on our patients. Prostate-specific antigen (PSA) response (PSA decline ≥ 50% from baseline), PSA progression-free survival, and overall survival (OS) were compared. Additionally, patients were further dichotomized according to predefined SUVmax thresholds different from those used in TheraP to analyze their potential impact on outcome as well. Results: In total, 107 mCRPC patients were included in this analysis (TheraP cePSMA PET–positive, n = 77; TheraP cePSMA PET–negative, n = 30). PSA response rates were higher in TheraP cePSMA PET–positive patients than in TheraP cePSMA PET–negative patients (54.5% vs. 20%, respectively; P = 0.0012). The median PSA progression-free survival (P = 0.007) and OS (P = 0.0007) of patients were significantly longer in the TheraP cePSMA PET–positive group than in the TheraP cePSMA PET–negative group. Moreover, being in the TheraP cePSMA PET–positive group was identified as a significant prognosticator of longer OS (P = 0.003). The application of different SUVmax thresholds for a single hottest lesion demonstrated no influence on outcome in patients eligible for PSMA RLT. Conclusion: Patient selection for PSMA RLT according to the inclusion criteria of TheraP led to a better treatment response and outcome in our preselected patient cohort. However, a relevant number of patients not fulfilling these criteria also showed substantial rates of response.
- metastatic castration-resistant prostate cancer
- mCRPC
- TheraP
- 68Ga-PSMA-11 PET
- prostate-specific membrane antigen targeted radioligand therapy
- PSMA RLT
In patients with metastatic castration-resistant prostate cancer (mCRPC), prostate-specific membrane antigen (PSMA) radioligand therapy (RLT) has emerged as a promising option with favorable efficacy and low toxicity and was recently approved by the Food and Drug Administration and the European Medicines Agency (1–4). Patients who received prior treatment usually undergo PET imaging (e.g., using 68Ga-PSMA-11) to assess for sufficient PSMA ligand uptake (5). To date, the criteria used to select patients are inconsistent in clinical use and even differ between prospective clinical trials (6).
Recently, the prospective, multicenter, randomized phase II TheraP study was published comparing 177Lu-PSMA-617 with cabazitaxel in 200 mCRPC patients (7). It reported a significantly higher treatment response and less toxicity in patients receiving 177Lu-PSMA-617. This trial used strict PSMA ligand PET–based selection criteria requiring high 68Ga-PSMA-11 tumor uptake with an SUVmax of at least 20 for at least 1 metastatic site, an SUVmax of greater than 10 for all other measurable (diameter, ≥10 mm) lesions, and absence of 18F-FDG–positive, PSMA ligand–negative tumor lesions (7). 177Lu-PSMA-I&T is another PSMA ligand showing promising results for therapy of mCRCP and is currently being explored in a prospective, multicenter, randomized phase III trial on mCRPC prior chemotherapy (SPLASH, NCT04647526) after second-line hormonal treatment (3,8). However, with the first results being expected in 2023, the PSMA PET avidity criteria required in this trial are so far unknown and cannot be addressed. High tumor uptake of 68Ga-PSMA-11 correlates with higher tumor radiation doses (9) and—despite not being proven for PSMA RLT—yields promise of better treatment effects. Therefore, following the theranostic paradigm, it seems reasonable to limit RLT to patients with high 68Ga-PSMA-11 tumor uptake and avoid including patients with a lower chance of response but still at risk for side effects. However, it remains unclear how well the selected SUVmax thresholds separate patients who do benefit from RLT from those do not, as biologic differences in the tumor might also play a substantial role.
Thus, the aim of this retrospective analysis was to evaluate the prognostic value of predefined SUVmax-based thresholds, including those applied in TheraP for the outcome of PSMA RLT. Outcome was measured by a prostate-specific antigen (PSA) decline of at least 50% from baseline, PSA progression-free survival (PFS), and overall survival (OS). Of note, the investigation included our large cohort of mCRPC patients previously treated with RLT using less restrictive criteria than in TheraP and therefore also encompasses patients who would not have been selected for RLT in this trial.
MATERIALS AND METHODS
Patients and 177Lu-PSMA-I&T RLT
From our institutional database of patients who underwent PSMA RLT using 177Lu-PSMA-I&T from December 2014 to July 2020 at the Department of Nuclear Medicine, School of Medicine, Technical University of Munich, 120 patients with 68Ga-PSMA-11 PET/CT imaging before treatment were screened, and 107 consecutive patients with PSMA PET imaging performed at our institution were selected. This patient population includes 73 patients for whom the safety and antitumor effect of PSMA RLT, but not the prognostic value of pretherapeutic 68Ga-PSMA-11 PET, have already been reported by Heck et al. (3). All patients had previously received second-line hormonal therapy with abiraterone or enzalutamide as well as chemotherapy or were unfit for chemotherapy. The patient characteristics are shown in Table 1. Before treatment, uptake in tumor lesions was confirmed by 68Ga-PSMA-11 PET imaging, and patients needed to present with lesions showing PSMA ligand uptake at least as high as liver background uptake. 177Lu-PSMA-I&T was synthesized and radiolabeled as reported by Weineisen et al. (10). 177Lu-PSMA-I&T was prepared according to good manufacturing practices and the German Medicinal Products Act (Arzneimittelgesetz §13 2b). In total, 444 cycles of PSMA RLT with a median of 4 cycles per patient (range, 2–20 cycles) were applied. Treatment was discontinued in patients with radiographic or clinical signs of progression or the appearance of severe toxicity according to the investigator. Patients received an intravenous treatment using a standard activity of 7.4 GBq of 177Lu-PSMA-I&T every 4–10 wk (median, 6 wk), which could be slightly adapted on the basis of, for example, lab test results and tumor burden. All patients gave written informed consent and were treated under the conditions of Declaration of Helsinki article 37, “Unproven Interventions in Clinical Practice.” The retrospective analysis was approved by the local ethics committee under reference number 115/18 S.
Baseline Patient Characteristics
Image Analysis and Definition of PET Eligibility
68Ga-PSMA-11 was synthesized according to Eder et al. (11). 68Ga-PSMA-11 was given to patients via an intravenous bolus followed by an intravenous injection of diuretic (furosemide). The PET acquisition began about 60 min after injection. All patients were examined on a Biograph mCT scanner (Siemens Medical Solutions). A diagnostic CT scan was initially performed in the portal venous phase 80 s after intravenous injection of an iodinated contrast agent (Imeron 300; Bracco Imaging) and was followed by the PET scan. All patients received a diluted oral contrast agent (300 mg of Telebrix; Guerbet). The PET scans were acquired in 3-dimensional mode with an acquisition time of 3–4 min per bed position or 1.1–1.5 mm/s using flow technique. Emission data were corrected for randoms, dead time, scatter, and attenuation and were reconstructed iteratively using ordered-subsets expectation maximization (4 iterations, 8 subsets) followed by a postreconstruction smoothing gaussian filter (5 mm in full width at half maximum). All patients were assessed as to whether they fulfilled the SUVmax-based criteria for 68Ga-PSMA-11 uptake of TheraP (TheraP contrast-enhanced PSMA [cePSMA] PET–positive vs. TheraP cePSMA PET–negative). The criteria from TheraP used for this analysis are shown in Table 2. In an additional analysis, we further explored the impact of the SUVmax threshold of 20 required to be fulfilled by at least 1 lesion in TheraP. For this analysis, exploratory thresholds between an SUVmax of 10 and an SUVmax of 50 were used, and the patients were restratified. The requirement of the SUVmax-based TheraP criteria of an SUVmax of at least 10 at all other sites of measurable (diameter, ≥10 mm) metastatic disease remained unchanged for this analysis. To determine the SUVmax of the tumor lesions, all were semiautomatically segmented using a predefined threshold and annotated regarding their malignancy (benign vs. malignant) and anatomic location (tissue type, organ) using the prototype software as described by Capobianco et al. (12). Our approach consisted of the following steps: first, all PSMA-avid foci with an SUVmax of at least 10 were automatically preselected, and foci with a PET volume smaller than 0.5 mL were discarded. Second, missed foci or foci falsely marked as physiologic were manually adjusted, if needed. Third, after the semiautomatic preselection of appropriate PSMA-avid foci, all 68Ga-PSMA-11 PET/CT scans were reread to assess whether they fulfilled the requirement of measurable metastatic disease.
PET-Based Eligibility Criteria
Notably, in comparison to TheraP, our patients do not undergo 18F-FDG PET before 177Lu-PSMA RLT. However, our institutional PET eligibility criteria (Table 2) require the use of contrast-enhanced CT, which, in comparison to 68Ga-PSMA-11 PET, can identify PSMA ligand–negative visceral and soft-tissue lesions. With this approach, only potential nonsclerotic PSMA-negative and 18F-FDG–positive disease might be missed.
Clinical Parameters, PSA Response, and PSA Progression
The following pretherapeutic parameters were collected and correlated with patient outcome: age, alkaline phosphatase, lactate dehydrogenase, hemoglobin, PSA, prior systemic therapies (including abiraterone, enzalutamide, first- and second-line chemotherapy, and 223Ra), lymph node–only metastases (N+/M1a), and visceral metastases (M1c). According to Prostate Cancer Clinical Trials Working Group 3, a PSA decline of at least 50% from baseline was defined as a PSA response (12). PSA progression was defined as either a PSA increase of at least 25% and at least 2 ng/mL above the nadir after an initial PSA decline or a PSA increase of at least 25% and at least 2 ng/mL from baseline in cases with no PSA decline (13).
Statistical Analysis
The primary outcome measures were PSA response, PSA PFS, and OS. The Kaplan–Meier method was used to estimate event time distributions, and log-rank tests were used for group comparisons. The frequencies of PSA response between the cePSMA PET–positive and cePSMA PET–negative groups within each SUVmax threshold group were compared using χ2 tests. Univariate and multivariate Cox regression analyses were performed to determine the association of pretherapeutic parameters with PSA PFS and OS. The corresponding hazard ratios (HRs) and 95% CIs are presented. A P value of less than 0.05 was considered statistically significant.
χ2 tests, Kaplan–Meier estimation, and log-rank tests were performed using Prism, version 8.4.3 (GraphPad Software), for Mac (Apple). Uni- and multivariate Cox regression analyses were performed using SPSS Statistics, version 25.0. (IBM Corp.), for Windows (Microsoft).
RESULTS
In total, 107 patients were analyzed. The median time on treatment was 4 mo (range, 1–57 mo). At baseline, lymph node, bone, and visceral metastases were present in 87 (81.3%), 97 (90.7%), and 31 (29.0%) patients, respectively. The median follow-up time was 11 mo (range, 1–63 mo). Forty-eight (44.9%) patients achieved a PSA response after PSMA-targeted RLT. Median OS and PSA PFS were 14.0 mo (95% CI, 11.0–17.0 mo) and 17.6 wk (95% CI, 14.6–29.7 wk), respectively. At the time of analysis, 88 patients showed PSA progression and 97 had died.
Clinical Outcome of TheraP cePSMA PET–Positive and TheraP cePSMA PET–Negative Patients
Seventy-seven (72%) patients were classified as TheraP cePSMA PET–positive, and 30 patients were classified as TheraP cePSMA PET–negative (28%; 9 patients with no metastatic lesion with an SUVmax ≥ 20, and 21 patients with ≥1 measurable metastatic lesion with an SUVmax < 10), who would not have been treated in TheraP on the basis of PSMA PET SUVmax criteria. Visceral metastases were present in 20 (26%) and 11 (37%) patients classified as TheraP cePSMA PET–positive and TheraP cePSMA PET–negative, respectively. Figure 1 compares the PET eligibility criteria used in TheraP with the retrospective stratification of patients treated in our compassionate-use program. Figures 2A–2D show an example of a TheraP cePSMA PET–positive patient and a TheraP cePSMA PET–negative patient.
Comparison of PET eligibility criteria used in TheraP (inner pie chart) and our retrospective stratification of patients (outer pie chart). In inner pie chart, green fill is patients who fulfilled PET eligibility criteria according to TheraP (TheraP–positive, 72%), gray fill is patients who were excluded because of discordant 18F-FDG–positive, PSMA-negative metastatic disease (18%), and red fill is patients who were not included because of low uptake on 68Ga-PSMA-11 PET/CT (SUVmax-negative, 10%). In outer pie chart (not drawn to scale), green is patients who retrospectively fulfilled inclusion criteria from TheraP (TheraP cePSMA PET–positive, 72%), and red is patients who retrospectively did not fulfill PSMA ligand PET-based SUVmax inclusion criteria (TheraP cePSMA PET–negative, 28%). Asterisk in gap represents patient cohort that was excluded from TheraP on basis of 18F-FDG–positive, PSMA-negative disease and was also not treated with PSMA RLT at our institution because of PSMA-negative visceral or soft-tissue lesions. Hatched areas represent patients in our cohort who were treated with PSMA RLT but might have been excluded from TheraP because of 18F-FDG–positive, PSMA-negative bone disease. ⊕ = positive; ⊖ = negative.
Examples of 68Ga-PSMA-11 PET/CT in mCRPC patients. (A and B) Maximum-intensity projection (A) and PSMA ligand PET/CT (B, top) and corresponding CT dataset (B, bottom) in 67-y-old patient from TheraP cePSMA PET–negative group with bone and lymph node metastases presenting with retrocrural lymph node metastasis with short-axis diameter of 14 mm and SUVmax of 8.1 (arrows). (C and D) Maximum-intensity projection (C) and PSMA ligand PET/CT (D, top) with corresponding CT dataset (D, bottom) in 74-y-old patient from TheraP cePSMA PET–positive group with bone and lymph node metastases presenting with retrocrural lymph node metastasis with short-axis diameter of 11 mm and SUVmax of 10.8 (arrows). PSA PFS and OS were 11 wk and 8 mo, respectively, in patient shown in A and B and 45 wk and 45 mo, respectively, in patient shown in C and D.
PSA response was achieved by 54.5% (n = 42) and 20% (n = 6) of patients in the TheraP cePSMA PET–positive and TheraP cePSMA PET–negative groups, respectively (P = 0.0012). A PSA waterfall plot (Fig. 3) shows the correlation between TheraP SUVmax criteria and the best PSA response. The median PSA PFS and OS were 6.0 versus 3.2 mo, respectively (HR, 0.5; 95% CI, 0.3–0.8; P = 0.007; Fig. 4A), for the TheraP cePSMA PET–positive group and 15.0 versus 10.0 mo, respectively (HR, 0.4; 95% CI, 0.2–0.7; P = 0.0007; Fig. 4B) for the TheraP cePSMA PET–negative group.
Waterfall plot showing response to treatment as measured by serum PSA. Color-coded best PSA response is defined as smallest increase or greatest decrease in PSA from baseline. Green indicates patients who fulfilled PET eligibility criteria (TheraP cePSMA PET–positive, n = 77). Red indicates patients who did not fulfill TheraP PET-based inclusion criteria (TheraP cePSMA PET–negative, n = 30). Asterisks indicate patients with increase of more than 100% in best PSA response.
Kaplan–Meier survival curves for PSA PFS (A) and OS (B) in TheraP cePSMA PET–positive and TheraP cePSMA PET–negative patients.
Both univariate and multivariate Cox regression analyses indicated that a TheraP cePSMA PET–positive status is a significant positive prognosticator for OS (P = 0.001 and P = 0.003 for uni- and multivariate analysis, respectively; Table 3). On univariate analysis, further parameters associated with worse OS were rising levels of lactate dehydrogenase and PSA, decreasing levels of hemoglobin, and the presence of visceral metastases at baseline PET (Table 3). In the multivariate Cox regression model, only rising lactate dehydrogenase, decreasing hemoglobin, and the presence of visceral metastases remained independent predictors of poor OS apart from the TheraP cePSMA PET–positive status (Table 3).
Uni- and Multivariate Cox Regression Analysis
Correlation Between Adapted SUV Thresholds for the Hottest Lesion on Clinical Outcome
When adjusting the SUVmax threshold required for at least 1 lesion without other changes in the PSMA ligand PET–based stratification, we obtained the following numbers of patients in the respective cePSMA PET–positive groups: 78 for an SUVmax of more than 10 or 15, 72 for an SUVmax of more than 25, 63 for an SUVmax of more than 30, 56 for an SUVmax of more than 35, 51 for an SUVmax of more than 40, and 47 for an SUVmax of more than 45 or 50 (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org).
The results on the PSA response, PSA PFS, and OS of the exploratory cePSMA PET–positive and cePSMA PET–negative groups using SUVmax thresholds of between 10 and 50 are presented in Supplemental Table 1. PSA responses significantly differed between the cePSMA PET–positive and cePSMA PET–negative groups when the threshold SUVmax for the hottest lesions was between 10 and 35 (all P < 0.05).
Further, the relative risk of death for the exploratory cePSMA PET–positive group showed a decreasing trend from a high to low adjusted SUVmax. It was lowest for an SUVmax of 20 (HR, 0.4; 95% CI, 0.3–0.9), followed by an SUVmax of 10, 15, 25, and 30 (HR, 0.5; 95% CI, 0.3–0.8 each) (Supplemental Table 1). OS significantly differed between the cePSMA PET–positive and cePSMA PET–negative groups with an exploratory SUVmax of between 10 and 35 (all P < 0.05; Figs. 5A–5D).
Kaplan–Meier survival curves for PSA PFS and OS in exploratory cePSMA PET–positive and exploratory cePSMA PET–negative patients stratified according to presence of PSMA-positive disease with SUVmax of at least 10 and 15 (A), 25 (B), 30 (C), 35 (D), 40 (E), and 45 and 50 (E).
The different exploratory SUVmax thresholds had no substantial effect on PSA response (range, 52.8%–55.6%) or median OS (range, 15.0–18.5 mo) in the group of cePSMA PET–positive patients. In the different exploratory cePSMA PET–negative groups, PSA response (range, 17.2%–38.3%) and median OS (range, 9.5–12.0 mo) declined in lower SUVmax thresholds (Supplemental Table 1).
DISCUSSION
Our retrospective analysis indicates that quantitative thresholds for PSMA ligand PET used in TheraP are predictive for response in patients treated with 177Lu-PSMA RLT selected on the basis of visual 68Ga-PSMA-11 uptake. Patients who fulfilled these criteria showed higher rates of maximum PSA response (54.5% vs. 20%, P = 0.0012), significantly longer PSA PFS (median, 6.0 vs. 3.2 mo, P = 0.007), and OS (median, 15.0 vs. 10.0 mo, P = 0.0007). Further, multivariate Cox regression analysis identified PSMA ligand PET–based criteria from TheraP (TheraP cePSMA PET–positive) as a new prognosticator for outcome in addition to known variables. In an additional exploratory analysis, adjustment of the SUVmax threshold required for the hottest lesion did not further select for higher response in the group fulfilling these criteria.
In TheraP, a maximum PSA decline of at least 50% was achieved in 66% of patients receiving 177Lu-PSMA RLT, compared with 54.5% in our TheraP cePSMA PET–positive cohort. The corresponding OS in TheraP patients was 19.1 mo, compared with 15.0 mo in our TheraP cePSMA PET–positive cohort (14). The slight shift toward a lower PSA response rate and shorter OS in our analysis might be explained by the more advanced disease stage in our TheraP cePSMA PET–positive cohort (visceral metastases in 26% of TheraP cePSMA PET–positive patients [n = 20] vs. 7% in TheraP patients [n = 7]), given the known negative association of visceral metastases with outcome (15). Another possible contributor might be the inclusion of patients with nonsclerotic PSMA-negative and 18F-FDG–positive bone disease in our TheraP cePSMA PET–positive cohort. We do not perform 18F-FDG PET at treatment selection for 177Lu-PSMA RLT, yet we strongly believe that our approach including contrast-enhanced CT within the PSMA ligand PET/CT reliably identifies PSMA-negative visceral lesions that are potentially 18F-FDG PET–positive. Consequently, this type of disease does not constitute a further confounder in our data compared with TheraP. Our institutional approach is also supported by recent results from Seifert et al., who reported a substantial level of agreement in findings between PSMA ligand PET/CT and combined 18F-FDG PET and PSMA ligand PET/CT for the assessment of therapy eligibility according to the VISION inclusion criteria (16). Although 18F-FDG PET and PSMA PET provide complementary information, in only 5% of patients was incremental information derived from dual-tracer PET/CT imaging (16). However, in a recently published analysis by Buteau et al., an increased 18F-FDG tumor volume (metabolic tumor volume ≥ 200 mL) in TheraP participants was significantly associated with lower rates of a maximum PSA decline of at least 50% (OR, 0.44; P = 0.01) and significantly correlated with a shorter PSA PFS (HR, 1.44; 95% CI, 1.28–2.52; P = 0.03) (17). Furthermore, in a retrospective analysis on patients who underwent 177Lu-PSMA, the median OS was significantly shorter (6.0 mo) in patients with discordant 18F-FDG–avid disease than in those without any 18F-FDG–positive, PSMA-negative lesions (16.0 mo) (18). This finding further underpins the potential prognostic value of combined 18F-FDG–negative, PSMA-negative PET imaging for treatment selection.
Sufficient PSMA ligand uptake of metastases in pretherapeutic PET is a prerequisite before PSMA RLT. However, no consensus on what should be considered sufficient exists (19). It is hypothesized that higher 68Ga-PSMA-11 uptake correlates with higher absorbed doses of 177Lu-PSMA, resulting in a favorable treatment response (9). Thus, it seems reasonable to restrict RLT to patients presenting with high 68Ga-PSMA-11 tumor uptake to increase treatment response and avoid unnecessary side effects in patients unlikely to respond. In our exploratory analysis using different SUVmax thresholds between 10 and 50 for the hottest lesion, no clear trend on patient outcome as measured by PSA response (range, 52.8%–55.6%) or OS (range, 15.0–18.5 mo) in the cePSMA PET–positive group was observed (Supplemental Table 1). This observation is in line with results from Seifert et al., who demonstrated no significant correlation between the highest SUVmax in a single lesion and OS (20). Similarly, Ferdinandus et al. found no significant correlation between uptake in pretherapeutic PET (SUVmax of different types of metastases and different tumor-to-normal organ ratios) (21).
In the cePSMA PET–negative group, outcome measurements tend to be worse in the lower than higher SUVmax threshold groups. PSA response ranged from 17.2% to 38.3% and median OS from 9.5 to 12.0 mo using SUVmax thresholds from 10 to 50 (Supplemental Table 1). One possible explanation could be that at decreasing SUVmax thresholds for the hottest lesion, the cePSMA PET–negative group contains a higher rate of patients with lesions below an SUVmax of 10. For example, at SUVmax thresholds of 50, 35, and 10 for the hottest lesion, 14 of 60, 21 of 30, and 29 of 29 patients, respectively, were classified as cePSMA PET–negative based on measurable lesions with an SUVmax of below 10 (Supplemental Table 1). This demonstrates an increasing selection of patients with a generally lower lesion uptake in the cePSMA PET–negative group, potentially explaining the lower response. Thus, a hypothesis might be that insufficient PSMA ligand uptake in lesions in general might be more relevant for PSMA RLT outcome than a single hottest lesion. This hypothesis is also supported by a substudy from Kuo et al. investigating the association between imaging parameters from baseline 68Ga-PSMA-11 PET/CT scans in the 177Lu-PSMA-617 arm of the VISION trial and clinical outcome (22). In this study, no significant correlation between SUVmax and treatment response or OS was found. However, a rising whole-body SUVmean correlated with survival and treatment response, supporting our hypothesis that insufficient PSMA ligand uptake in lesions in general might play a crucial role for the outcome of PSMA RLT. This result is also in line with results from Gafita et al. demonstrating rising values of tumor SUVmean to be significantly correlated with better outcome (23).
There are several limitations to our analysis, including its retrospective nature. In addition, although we could assess the impact of the TheraP criteria used in 68Ga-PSMA-11 PET on treatment outcome, our cohort did not undergo additional 18F-FDG PET. Thus, exact comparison with the TheraP cohort is not possible. Nevertheless, we believe that our approach including contrast-enhanced CT in the pretherapeutic workup selects most 18F-FDG–positive, PSMA-negative disease, as discussed.
CONCLUSION
The results of our analysis demonstrate a better treatment response and outcome in mCRPC patients who underwent 177Lu-PSMA RLT and retrospectively fulfilled the 68Ga-PSMA-11–based TheraP inclusion criteria. However, as a relevant number of patients not fulfilling these stricter criteria also showed substantial rates of response, it remains to be discussed whether PSMA RLT needs to be withheld from these patients. Finally, our exploratory analyses using different SUVmax thresholds for the hottest lesion indicate that this criterion in TheraP is less prognostic than insufficient PSMA ligand uptake of lesions in general.
DISCLOSURE
Matthias Eiber reports fees from Blue Earth Diagnostics Ltd. (consultant, research funding), Novartis/AAA (consultant), Telix (consultant), Bayer (consultant, research funding), RayzeBio (consultant), Point Biopharma (consultant), Janssen Pharmaceuticals (consultant, speakers’ bureau), Parexel (image review), and Bioclinica (image review) outside the submitted work and a patent application for rhPSMA. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is retrospective application of different TheraP PET-based inclusion criteria in mCRPC patients treated with RLT associated with higher rates of maximum PSA decline of at least 50%, and does it correlate with longer PSA PFS and OS?
PERTINENT FINDINGS: Retrospective application of the criteria was associated with higher rates of maximum PSA decline of at least 50% and significantly correlated with longer PSA PFS and OS.
IMPLICATIONS FOR PATIENT CARE: The 68Ga-PSMA-11–based PET selection criteria used in TheraP are highly prognostic of better treatment outcome. The SUVmax threshold for the hottest lesions seems to be less relevant than insufficient PSMA ligand uptake in lesions in general.
Footnotes
Published online Jun. 8, 2023.
- © 2023 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication December 19, 2022.
- Revision received March 24, 2023.