Abstract
We have previously reported that PET using 18F-fluoride (NaF PET) for assessment of osseous metastatic disease was associated with substantial changes in intended management in Medicare beneficiaries participating in the National Oncologic PET Registry (NOPR). Here, we use Medicare administrative data to examine the association between NaF PET results and hospice claims within 180 d and 1-y survival. Methods: We classified NOPR NaF PET results linked to Medicare claims by imaging indication (initial staging [IS]; detection of suspected first osseous metastasis [FOM]; suspected progression of osseous metastasis [POM]; or treatment monitoring [TM]) and type of cancer (prostate, lung, breast, or other). Results were classified as definitely positive scan findings versus probably positive scan findings versus negative scan findings for osseous metastasis for IS and FOM; more extensive disease versus no change or less extensive disease for POM; and worse prognosis versus no change or better prognosis for TM, based on the postscan assessment. Our study included 21,167 scans obtained from 2011 to 2014 of consenting NOPR participants aged 65 y or older. Results: The relative risk of hospice claims within 180 d of a NaF PET scan was 2.0–7.5 times higher for patients with evidence of new or progressing osseous metastasis than for those without, depending on indication and cancer type (all P < 0.008). The percentage difference in hospice claims for those with a finding of new or more advanced osseous disease ranged from 3.9% for IS prostate patients to 28% for FOM lung patients. Six-month survival was also associated with evidence of new or increased osseous disease; risk of death was 1.8–5.1 times as likely (all P ≤ 0.0001), with percentage differences of approximately 30% comparing positive and negative scans in patients with lung cancer imaged for IS or FOM. Conclusion: Our analyses demonstrated that NaF PET scan results are highly associated with subsequent hospice claims and, ultimately, with patient survival. NaF PET provides important information on the presence of osseous metastasis and prognosis to assist patients and their physicians when making decisions on whether to select palliative care and transition to hospice or whether to continue treatment.
See an invited perspective on this article on page 418.
The National Oncologic PET Registry (NOPR) was established in response to the Centers for Medicare & Medicaid Services (CMS) Coverage with Evidence Development policy. Initially, NOPR focused on evaluating the impact of PET with 18F-FDG (1,2). Information on intended patient management was collected prospectively from the referring physician before and after PET. These results are reported elsewhere (3–5). In 2011, NOPR was extended to examine whether 18F-fluoride PET (NaF PET), used for detection of osseous metastatic disease, has an impact on patient management. Although 18F-fluoride was initially approved by the U.S. Food and Drug Administration in 1972, its use became uncommon after the development of 99mTc radiopharmaceuticals for conventional bone scintigraphy (6). Recently, interest in the use of NaF PET as an alternative to conventional bone scintigraphy has increased because of evidence indicating the superior diagnostic performance of NaF PET (7), and because of worldwide shortages of the 99Mo used for generator production of 99mTc (8). The results of NOPR for NaF PET reported in 2014 and 2015 showed that, like 18F-FDG PET, this diagnostic test also had a substantial impact on the intended management of patients with suspected or known osseous metastatic disease (9–11).
The findings of the NOPR have been subject to 2 primary criticisms—first, that changes in planned management are only a surrogate and do not document actual care delivered, and second, that the imaging results were not linked to patient outcomes (12–15). These issues were noted in the 2015 CMS National Coverage Decision in response to a request to cover NaF PET. CMS highlighted the failure of the NOPR results to demonstrate that use of NaF PET led to more appropriate palliative or curative care, improved quality of life, or improved survival (16).
To address these concerns in part, the NOPR investigators requested Medicare claims available through the CMS Virtual Data Research Center for consenting NOPR participants (17). We used these data to evaluate the association between NaF PET results or the referring physician’s post-PET assessment of disease extent or patient prognosis (collected by NOPR) and subsequent hospice claims and survival (recorded in the Virtual Data Research Center). In these analyses, we infer that associations between PET results and hospice claims within 180 d and survival at 180 d and 1 y are indirect indicators of use of NaF PET findings to implement appropriate palliative care. We hypothesized that NaF PET detection of new osseous metastatic disease or of worsening metastatic disease would lead to more timely transfer to hospice and the availability of accurate information for patients on their expected prognosis.
MATERIALS AND METHODS
NOPR is a prospective data registry (ClinicalTrials.gov NCT00868582), the operational details and findings of which have been previously reported (2–5,9–11). The NaF PET registry opened on January 31, 2011, and the first patient was registered on February 7, 2011. The registry was closed to accrual on December 14, 2017.
Collection of Data Under Coverage with Evidence Development in NOPR
Physicians referring Medicare patients for NaF PET scans were required to complete forms describing the planned management of their patients before and after the PET scans. Interpreting physicians also completed a PET assessment form documenting scan results.
Construction of Study Cohort
Consent for the research use of NOPR data was requested from patients, as well as from both referring and interpreting physicians; overall consent was obtained in approximately 85% of cases. For the linkage of NOPR data with Medicare claims, the analysis cohort consisted of patients registered on or after February 7, 2011, who underwent NaF PET by September 30, 2014, allowing at least 90 d for claims to appear in the 2011–2014 Medicare claims data available in the Virtual Data Research Center. We restricted our analyses to patients aged 65 y or older, as that was the population used in our prior reports.
Medicare Data
A finder file of NOPR participants including social security numbers, birth date, and sex was provided to CMS. The linked Medicare claims data were placed in the Virtual Data Research Center analytic space. For these analyses, it did not matter if the participant was in traditional fee-for-service Medicare or a Medicare Advantage plan, as hospice claims and date of death were available for both patient subsets (18). We focused on hospice claims within 180 d (information on the first date of hospice care was inferred from the claims data in the hospice research identifiable file) and survival at 180 d and 1 y (based on date of death from the Master Beneficiary Summary File) after each patient’s NaF PET study.
NOPR Analysis Dataset
We used selected information from the NOPR data stored at Brown University, including scan date, testing indication, cancer type, and scan findings. For the purposes of this analysis, we used information from the pre-PET form documenting the cancer type and the indication for the scan. Scan indications were recorded as initial staging of newly diagnosed cancer (IS), suspected first osseous metastasis (FOM) of a previously treated cancer, suspected new osseous metastasis as a site of disease progression or progression of osseous metastasis (POM), or treatment monitoring (TM). Within each indication, we stratified our analyses by cancer type as prostate, lung, breast, and all others, if the number of observations was large enough to be compliant with the CMS prohibition on the display of cell sizes less than 11 (19). In instances of sparse data, we included breast or lung cancers in the other-cancers group.
An individual patient could have had more than one NaF PET scan. We used the first scan reported for IS. For subsequent treatment strategy indications (FOM, POM, TM), if there was more than one scan, we used the last scan done for each indication, as we wanted to use the scan most likely to be associated with stopping active therapy, hospice use, and survival.
The interpreting physician’s PET assessment form recorded whether the scan was normal or had only benign skeletal abnormalities versus osseous metastatic disease that was unifocal, multifocal, or diffuse, as well as the level of confidence that the findings were definite, probable, or equivocal for metastasis. For the IS and FOM analyses, these scan findings were combined into 3 categories: negative (normal, benign or equivocal), probably positive, and positive (definite osseous metastasis, including unifocal, multifocal, or diffuse disease).
For analyses of POM and TM, the categorization of scan results was modified to reflect the presence or absence of disease progression. For POM, we used the referring physician’s summary assessment that scan findings indicated more extensive disease, no change, or less extensive disease. We focused on the comparison of more extensive disease (progression) versus no change or less extensive disease. For TM, we relied on the referring physician’s assessment of patient prognosis based on the scan results as better, no change, or worse. We focused on the comparison of worse versus better or no change. For both POM and TM, we included patients with probably-positive scans (per the radiologist interpretation), in the appropriate categories per the referring physician’s assessment.
Statistical Analyses
For each indication–cancer combination, we ascertained the proportion of patients admitted to hospice within 180 d or dying within 180 d after NaF PET by the scan results or clinical assessments described above. Within each result strata, we estimated the risk ratio and the difference in percentage, comparing risk of hospice claims within 180 d and risk of death within 180 d. Significance was assessed using the χ2 test. We also conducted a time-to-event analysis (for 180 d for hospice claims and 365 d for death) by generating Kaplan–Meier curves. We compared survival distributions across the scan results using the log-rank test.
A P value threshold of 0.05 was used to signify statistical significance. Statistical analyses were performed using SAS 9.4 (SAS Institute). When interpreting our results, we adjusted for multiple comparisons by using the Bonferroni adjustment approach.
RESULTS
Table 1 shows the source population and exclusion criteria used to create our final analytic dataset from the 32,663 NaF PET scans obtained during the study period. Of these scans, 4,648 (14%) were for patients who did not consent to participate in research (or whose physicians did not consent), 1,520 were for patients younger than 65 y, and 652 could not be matched in the Medicare dataset. There were 21,167 scans available for analysis. FOM was by far the most common indication for NaF PET (n = 10,270), followed by IS (n = 5,155), POM (n = 2,655), and TM (n = 3,087).
Selection of Cases for Inclusion in Cohort
The characteristics of the study population are shown in Table 2. The median age was 74 y. Many patients were asymptomatic (26.2%) or were referred because of a rising level of prostate-specific antigen (30.0%). Over 45% were considered to have local or regional disease at the time of NaF PET.
Demographics of Study Cohort (n = 21,167)
The number and percentage of patients with hospice claims within 180 d of NaF PET stratified by indication for imaging, cancer type, and scan result are shown in Table 3. Patients with positive scans (for IS and FOM) were consistently more likely to have a hospice claim within 180 d of the scan than those with negative scans, regardless of cancer type. For patients being assessed for IS, the range was from a low of 2.0 times more likely for patients with other cancers to a high of 6.7 times more likely for patients with prostate cancer (P < 0.008 for all comparisons). The corresponding percentage differences were also large comparing patients with positive versus negative scans, ranging from a 3.9% higher risk of hospice claims for prostate cancer patients to 23.6% for lung cancer patients. Patients evaluated for FOM were between 2.6 and 7.5 times more likely to have a hospice claim after positive scans than after negative scans, depending on cancer type (P < 0.0001, for all comparisons). The percentage difference in hospice claims for patients with lung cancer evaluated for FOM who had positive versus negative scans was nearly 30% higher, whereas the difference was 17.1% for other-cancer patients and 6.7% and 8.0% for breast and prostate cancer patients. Patients scanned for both IS and FOM whose scans were probably positive had intermediate results. Patients scanned to assess POM who were found to have more extensive disease were 1.9–2.5 times more likely to have a hospice claim within 180 d (P < 0.0003, for all comparisons) than were those with no change or less extensive disease. This translated into a 10% increase in the risk of hospice claims, regardless of cancer type, for patients with more extensive disease as opposed to no change or less extensive disease on NaF PET. Patients scanned for TM were 2.2–2.6 times more likely to have a hospice claim within 180 d if the scan showed a worse prognosis than if it showed no change or a better prognosis (P < 0.0001 for all comparisons). Patients with a worse prognosis had a 9.4%–13.8% higher risk of hospice claims than did those with no change or a better prognosis. All comparisons, except for IS of other-cancer patients, were significant at the 0.05 level after Bonferroni adjustment.
Hospice Claims and Deaths Within 180 Days of NaF PET, by Indication for Imaging, Cancer Type, and Scan Result
Table 3 also shows the number and percentage of patients who died within 180 d of NaF PET stratified by indication for imaging, cancer type, and scan result. Patients imaged for IS were 1.7–5.1 times more likely to die within 180 d if the scan was positive than if it was negative, depending on tumor type (P ≤ 0.0001 for all comparisons). The percentage difference in deaths for patients with positive as opposed to negative scans ranged from 4.7% higher for prostate cancer patients to 35.8% higher for lung cancer patients. Patients imaged for FOM with a positive scan were 2.2–5.0 times more likely to die within 180 d after the scan than were those with a negative scan, depending on cancer type (P ≤ 0.0001 for all comparisons). Percentage differences in 180-d survival for patients with a positive as opposed to a negative FOM scan were about 7%–8% for prostate and breast cancers, 33.7% for lung cancer, and 17.2% for other cancers.
The survival results were similar for those with scans done for POM and TM. For POM, if the scans showed more extensive disease, the relative risk of death was 2.1 for prostate cancer and 1.8 for other cancers (P < 0.0001 and P = 0.0005, respectively). In patients imaged for disease progression, the percentage difference exceeded 9% for death at 180 d in patients with more extensive disease versus patients with no change or less extensive disease, regardless of cancer type. In patients imaged for TM, evidence of a worse prognosis, as opposed to no change or a better prognosis, was also associated with a higher relative risk of death, 2.3 for prostate and other cancers (P < 0.0001 for both comparisons). The percentage difference for those with a better prognosis versus those with no change or a worse prognosis was over 9%. All comparisons were significant at the 0.05 level after Bonferroni adjustment.
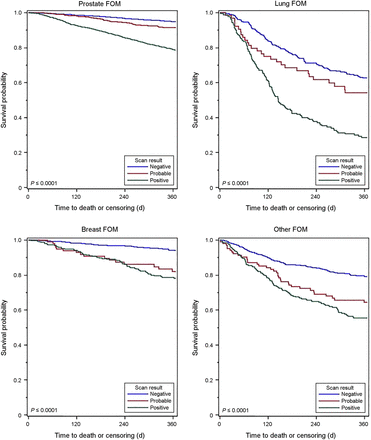
Figures 1– 3 show the Kaplan–Meier 1-y survival curves after NaF PET. Supplemental Figures 1–3 (available at http://jnm.snmjournals.org) show the time to hospice claims within 6 mo of the scan. The predictive value of NaF PET is evident in these curves as well. The information presented in the survival curves is consistent with that in the tables both for hospice claims and for survival, and the predictive nature of NaF PET was evident across all imaging indications and cancer types. In all cases, time to hospice and time to death were substantially shorter if the scan was positive (IS, FOM) than if negative, and if the scan showed evidence of more extensive disease (POM) or worse prognosis (TM) than the same/less extensive disease or no change/better prognosis.
Kaplan–Meier curves demonstrating 1-y survival after NaF PET performed for initial staging (IS) of prostate, lung, and other cancers for patients with positive, probably positive, or negative scans. Log-rank test P values are shown.
Kaplan–Meier curves demonstrating 1-y survival after NaF PET performed for detection of suspected FOM of prostate, lung, breast, and other cancers for patients with positive, probably positive, or negative scans. Log-rank test P values are shown.
Kaplan–Meier curves demonstrating 1-y survival after NaF PET performed for suspected POM of known osseous metastasis and for TM of prostate and other cancers. Curves are dichotomized on the basis of referring physician assessment after PET that patient has more extensive disease vs. no change or less extensive disease (POM) or that prognosis is worse vs. unchanged or better (TM). Log-rank test P values are shown.
DISCUSSION
In our cohort of older cancer patients who underwent NaF PET, a positive scan result was highly associated with hospice claims and survival for all indications and cancer types. The size of the effect modestly differed by cancer type. The starkest differences occurred among lung cancer patients. Our findings that positive NaF PET assessed qualitatively is associated with lower survival rates are consistent with other published reports that have focused on the association between tumor burden assessed quantitatively by NaF PET and survival (20–24). We could find no similar research on admission to hospice. The analysis of NOPR NaF PET data linked with Medicare claims data is strengthened by the use of actual outcome data from Medicare claims for the patients imaged under coverage with evidence development.
Only a few small, single-center studies have evaluated the association between NaF PET results and cancer survival (20–24). The relative survivals reported in these studies were similar to those reported here, except for 2 studies (22,24) that reported differences of lesser magnitude, based on NaF PET results, than we found. This difference in results may be attributable to the extended follow-up time (3–5 y) in those studies, because with extended follow-up, the curves converge. In addition, those studies focused on quantitative indices of tumor burden, as opposed to our measures of positive versus negative scans, more extensive disease versus no change or less extensive disease, and worse prognosis versus no change or better prognosis. Our findings are likely to be more generalizable to common practice because our registry captured patients imaged in the course of standard medical practice, as opposed to in small, single-center studies at academic medical centers.
The proportion of patients who died within 1 y of a positive NaF PET scan was similar to that in published reports. In our cohort of patients imaged for IS, who were found to have osseous metastases, 12% of prostate cancer patients, 64% of lung cancer patients, and 31% of other-cancer patients had died within 1 y of the scan. In a Chinese cohort of cancer patients with bone metastases, Zhang et al. reported that 46% of lung cancer patients had died by the 1-year follow-up (25). The proportion dying within 1 y was higher in a large population-based Danish registry study that reported 90% of lung cancer patients and 35% of prostate cancer patients dead within a year of bone metastasis (26). The higher mortality rates, compared with those we report, are likely due to the initiation of follow-up at the documentation of first skeleton-related events, as opposed to first evidence of osseous metastasis detected by sensitive imaging methods.
The proportion of cancer patients (27), and prostate cancer patients in particular (28), who enter hospice is increasing, but the time spent in hospice is often short (28) and often preceded by intense medical care (27). During the 6 mo after their NaF PET scan, 204 patients (18%) in our POM prostate cancer cohort with evidence of more extensive disease died and 183 (16%) had hospice claims. Thus, on average, 90% (183/204) were in hospice. Similarly, within 6 mo of their scan, 114 (15%) of TM prostate cancer patients with a worse prognosis entered hospice and 124 (17%) died. The proportion of POM other-cancer patients with more extensive disease who entered hospice (22%) and who died (22%) was similarly high, as was the proportion of TM other-cancer patients who entered hospice (25%) and who died (29%). Although we cannot assume that all those who entered hospice died during the 6-mo period, the proportion who died in hospice appears to be somewhat higher than found in the literature. The data presented by Teno et al. (27) show an upward trajectory in the use of hospice in recent years. The use of NaF PET to detect osseous metastasis early promises to improve the proportion of patients who experience early referral to hospice, thus avoiding medically intensive care shortly before hospice entry.
Patients with cancers who have osseous metastatic disease confirmed on bone scintigraphy (including NaF PET) will receive more appropriate therapy, with the goal of that therapy directed to treatment or redirected to a palliative intent. Patients undergoing IS or evaluation for suspected FOM whose scans are negative are better candidates for curative or life-prolonging therapies, whereas those with positive scans, or evidence of progressing disease, may be effectively guided to appropriate palliative therapy, including hospice. Our data support that this is the case.
An important limitation of the NOPR is that it was a prospective observational study, and not a randomized clinical trial. Thus, we have no representative group of patients for comparison with those who underwent NaF PET. Accordingly, we acknowledge that we are unable to demonstrate that the patient outcomes we observed in association with NaF PET results are causally related fully or in part to the NaF PET findings.
Another limitation of our methods, as a result of the restrictions on display of cell counts less than 11, was our inability to stratify the data for specific, less frequent types of cancer in our cohort. However, we believe that the strength of the associations we report mitigates this issue.
CONCLUSION
Our analyses demonstrate that NaF PET scan results are highly associated with referral to hospice and, ultimately, with patient survival. The size of the associated differences noted here across indications and cancer types is compelling. The results of NaF PET provide a strong tool for guiding effective, appropriate planning of patient management. NaF PET provides important information on the presence of osseous metastasis to assist patients and their physicians when making decisions on admission to hospice and continued treatment.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
Acknowledgments
The NOPR is sponsored by the World Molecular Imaging Society, managed by the American College of Radiology, and self-supported by the fees paid by participating PET facilities.
Footnotes
Published online Dec. 28, 2017.
- © 2018 by the Society of Nuclear Medicine and Molecular Imaging.
REFERENCES
- Received for publication November 20, 2017.
- Accepted for publication December 20, 2017.