Abstract
18F-N-[3-bromo-4-(3-fluoro-propoxy)-benzyl]-guanidine (18F-LMI1195) is a new PET tracer designed for noninvasive assessment of sympathetic innervation of the heart. The 18F label facilitates the imaging advantages of PET over SPECT technology while allowing centralized manufacturing. Highly specific neural uptake of 18F-LMI1195 has previously been established, but the retention kinetics are not yet fully understood. Methods: Healthy New Zealand White rabbits were studied with 18F-LMI1195 using a small-animal PET system. Dynamic 40-min chest scans were started just before intravenous bolus injection of 18F-LMI1195. Imaging was performed under norepinephrine transport inhibition with desipramine pretreatment, a 1.5 mg/kg desipramine chase administered 10 min after tracer injection, and saline treatment of controls. As a reference, cardiac uptake of 11C-hydroxyephedrine and 123I-metaiodobenzylguanidine (123I-MIBG) was examined by PET and planar scintigraphy, respectively. Results: Cardiac uptake of all 3 tracers was inhibited by pretreatment with desipramine. Stable cardiac tracer retention was delineated by dynamic PET in control rabbits for 11C-hydroxyephedrine (washout rate, 0.42% ± 0.57%/min) and 18F-LMI1195 (washout rate, 0.058% ± 0.28%/min). A desipramine chase increased 11C-hydroxyephedrine washout from the heart (2.43% ± 0.15%/min, P < 0.001), whereas 18F-LMI1195 washout was not influenced (0.059% ± 0.11%/min, not statistically significant). Additionally, a desipramine chase did not change the cardiac 123I-MIBG uptake (delayed heart-to-mediastinum ratio, 1.99 ± 0.12 (desipramine chase) vs. 2.05 ± 0.16 (controls), not statistically significant). Conclusion: In vivo norepinephrine transporter (NET) blockade with desipramine confirmed specific neural uptake of 18F-LMI1195, 11C-hydroxyephedrine, and 123I-MIBG in rabbit hearts. 11C-hydroxyephedrine cardiac retention was sensitive to a NET inhibitor chase, indicating a cycle of continuous NET uptake and release at the nerve terminals. In contrast, 18F-LMI1195 and 123I-MIBG demonstrated stable storage at the nerve terminal with resistance to a NET inhibitor chase, mimicking physiologic norepinephrine turnover.
- 18F-LMI1195
- 11C-hydroxyephedrine
- 123I-metaiodobenzylguanidine
- positron emission tomography
- sympathetic nerve
The importance of the role that the sympathetic nervous system plays in various cardiac disorders, including ischemic heart disease and heart failure, has been well established (1). Radionuclide imaging provides a unique technique to noninvasively monitor the local condition of the cardiac sympathetic nervous system in patients. The SPECT imaging agent 123I-metaiodobenzylguanidine (123I-MIBG) is commercially available and has been demonstrated in clinical trials to provide important clinical information for risk stratification in heart failure patients (2). However, PET offers improvements over SPECT, namely increased spatiotemporal resolution and well-validated quantification approaches for tissue radiotracer concentrations. The 11C-labeled norepinephrine analog hydroxyephedrine is the most commonly used PET tracer for sympathetic nerve imaging. The superior sensitivity and quantitative capability of 11C-hydroxyephedrine PET allows for reliable assessment of regional neurohumoral abnormalities (3). However, the short half-life of 11C (20 min) limits widespread clinical use because of the need for a costly on-site cyclotron.
To overcome the limitations of these conventional tracers, the novel 18F-labeled PET imaging agent 18F-N-[3-bromo-4-(3-fluoro-propoxy)-benzyl]-guanidine (18F-LMI1195) has been developed (4). It inherits the advantages of 18F-radionuclides, including a longer physical half-life (110 min). An 18F-labeled radiotracer could significantly reduce the cost of tracer production by leveraging delivery systems similar to 18F-FDG used in clinical imaging. Furthermore, the longer half-life can provide more flexibility in study design, allowing for delayed or prolonged imaging. Yu et al. initially characterized 18F-LMI1195 for cardiac neuronal imaging (4). The high affinity and specificity of 18F-LMI1195 for the neuronal norepinephrine transporter (NET) were confirmed with a cell membrane binding assay. The feasibility of in vivo imaging with targeting of neuronal NETs at the presynaptic level was demonstrated using a combination of rabbits and nonhuman primate models. Furthermore, minimal liver uptake in comparison to other conventional nerve tracers was suggested in the animal experiments and is preferable for assessment of the left ventricular inferior wall. Most recently, a human volunteer study (clinical phase I trial) with 18F-LMI1195 demonstrated promising clinical results, with uniform myocardial tracer uptake in the left ventricular wall and acceptable radiation exposure (5). Further mechanistic details of 18F-LMI1195 cardiac retention are not yet fully elucidated. Understanding the uptake and retention kinetics at a subcellular level is essential in the design of clinical imaging protocols and in the interpretation of imaging results. In this study, we evaluated the in vivo cardiac retention kinetics of this novel PET sympathetic nerve tracer by directly comparing it with the conventional tracers 123I-MIBG and 11C-hydroxyephedrine in rabbit hearts (Fig. 1).
Chemical structures of the neurotransmitter norepinephrine, the sympathetic nerve radiotracer 18F-LMI1195, and the conventional tracers 123I-MIBG and 11C-hydroxyephedrine (11C-HED).
MATERIALS AND METHODS
Radiopharmaceuticals
The 123I-MIBG (AdreView; GE Healthcare) had a specific activity of 74 MBq (2 mCi)/mL and a radiochemical purity of more than 95%. 18F-LMI1195 and 11C-hydroxyephedrine were both synthesized following described procedures (4,6). Analyses at the end of syntheses revealed specific radioactivity greater than 10 GBq/μmol and radiochemical purity greater than 95% for both radiolabeled compounds.
Animal Studies
Healthy New Zealand White rabbits (Charles River Laboratories Inc.) weighing 2.3 ± 0.8 kg were used. The animal protocols were approved by the local institutional animal care and use committee and were conducted strictly according to the Guide for the Care and Use of Laboratory Animals (7). Group sizes corresponded to the biometrical calculations provided in the relevant animal license.
The animals were studied with 18F-LMI1195 PET, 11C-hydroxyephedrine PET, and 123I-MIBG planar scintigraphy under 3 different medications: intravenous pretreatment with the NET inhibitor desipramine, 1.5 mg/kg, 10 min before tracer injection (n = 3); a desipramine chase of 1.5 mg/kg, administered 10 min after tracer delivery (n = 4); and saline pretreatment of controls (n = 4).
Imaging Protocols
All animals were maintained under anesthesia with 2% isoflurane throughout the imaging procedure. Imaging with 18F-LMI1195 and 11C-hydroxyephedrine was performed using a dedicated small-animal PET system (Inveon; Siemens Healthcare). Its characteristics have been described previously (8,9). Just before a bolus tracer injection (11C-hydroxyephedrine, 50 MBq; 18F-LMI1195, 50 MBq) via the ear vein, a list-mode 40-min image acquisition was started. The list-mode data were reconstructed into a dynamic sequence (25 frames: 15 × 8 s, 3 × 60 s, 7 × 300 s) using ordered-subset expectation maximization with 16 subsets and 4 iterations. The reconstructed PET images were analyzed using an image-processing application (AMIDE-bin, version 1.0.2) (10). Regions of interest were drawn manually on the left ventricular wall, and decay-corrected time–activity curves were generated. The tracer washout rate (%/min) was calculated as follows: {([cardiac counts10 min – cardiac counts35 min]/cardiac counts10 min) × 100/25 (min)}.
Planar 123I-MIBG imaging was performed using a clinical dual-head γ camera (Symbia E; Siemens Healthcare) equipped with a medium-energy collimator. The animals were placed prone on one detector head. Ten-minute planar acquisitions were started 10 min (early) and 2.5 h (delayed) after 123I-MIBG (50 MBq) injection via the ear vein (128 × 128 matrices, a photopeak window set at 15% around 159 keV). Heart-to-mediastinum ratios were calculated by dividing the count density of the manually drawn region of interest on the left ventricle by that on the mediastinum.
Statistical Analysis
All results are displayed as mean ± SD. The 2-tailed paired Student t test was used to compare differences between 2 dependent groups, and the 2-tailed independent Student t test was used to compare differences between independent groups. A P value of less than 0.05 was assumed to be statistically significant. Statistical analysis was done with StatMate III (ATMS Co., Ltd.).
RESULTS
Cardiac Uptake of 18F-LMI1195
18F-LMI1195 PET images of control animals demonstrated clear visualization of the left ventricular wall, with high and homogeneous tracer activity seen throughout the left ventricular myocardium (Fig. 2). Uptake in the surrounding organs, including the liver, remained low compared with the myocardium at 10 and 35 min after tracer injection. Pretreatment with the neural NET inhibitor desipramine reduced cardiac uptake dramatically, and almost no cardiac uptake was identified. In the same way, 11C-hydroxyephedrine cardiac uptake throughout the ventricle was seen in controls and was diminished with desipramine pretreatment. 123I-MIBG planar images also showed focally increased tracer accumulation in the heart, which desipramine pretreatment reduced on the delayed scans (Fig. 3).
Averaged time–activity curves and representative short-axis images of in vivo rabbit cardiac PET imaging. Desipramine (DMI) chase enhanced 11C-hydroxyephedrine (11C-HED) washout from heart, whereas 18F-LMI1195 washout did not change.
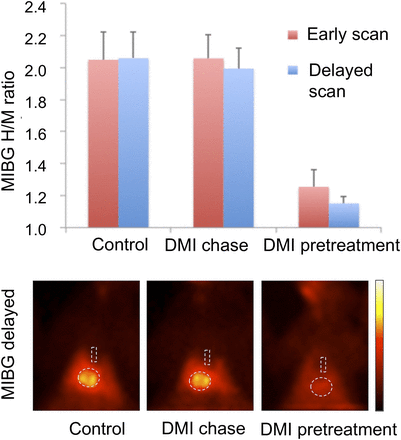
Results of in vivo rabbit 123I-MIBG planar scintigraphy of chest. Desipramine (DMI) chase did not change cardiac distribution of 123I-MIBG. Dotted lines indicate regions of interest in heart and mediastinum. H/M ratio = heart-to-mediastinum ratio.
Cardiac Retention of 18F-LMI1195
18F-LMI1195 retention in the heart was stable in controls (washout ratio, 0.058% ± 0.28%/min). A NET inhibitor chase with desipramine, added only after initial tracer accumulation, did not change cardiac tracer washout (0.059% ± 0.11%/min, not statistically significant) (Fig. 2). On the other hand, cardiac washout of 11C-hydroxyephedrine was slightly higher than that of 18F-LMI1195 (0.42% ± 0.57%/min) in controls, and washout was further increased by the NET inhibitor chase (2.43% ± 0.15%/min, P < 0.001). 123I-MIBG cardiac activity was not influenced by the desipramine chase (delayed heart-to-mediastinum ratio; control and desipramine chase, 2.05 ± 0.16 and 1.99 ± 0.12, respectively, not statistically significant) (Fig. 3).
DISCUSSION
In the present study, we directly compared the uptake kinetics of a new sympathetic nerve tracer, 18F-LMI1195, with the most widely used conventional PET and SPECT tracers (11C-hydroxyephedrine and 123I-MIBG) using pharmacologic interventions with the NET inhibitor desipramine. All tested tracers demonstrated feasibility for neural imaging in rabbit hearts, with specific initial uptake via the NET. Interestingly, desipramine—added only after initial tracer distribution as a NET inhibitor chase treatment—demonstrated distinct differences among the tracers (Fig. 4). The 11C-hydroxyephedrine washout from the heart was enhanced by the desipramine chase, suggesting that continuous cyclical release (diffusion out) and reuptake of 11C-hydroxyephedrine via neural NET is occurring to maintain the tracer retention in the heart. In contrast, 18F-LMI1195 and 123I-MIBG cardiac retentions were not influenced by the desipramine chase, demonstrating stable tracer retention in the nerve terminals independent of NET activity after initial transport. Although further confirmation in humans is needed, these basic characteristics of tracer kinetics may be helpful for optimizing clinical protocols and for interpreting imaging results. Furthermore, with an understanding of these basic tracer kinetics, a clinical interpreter could select or even combine more than 2 tracers to gain insight into the specific processes of norepinephrine handling in the nerve terminals (11).
Simplified schematic of 11C-hydroxyephedrine (11C-HED), 123I-MIBG, and 18F-LMI1195 kinetics at nerve terminals. After transportation into nerve terminals via uptake-1 NET system, 11C-hydroxyephedrine retention is maintained by continuous uptake-1 activity, whereas 18F-LMI1195 and 123I-MIBG seem to be stored stably at nerve terminals.
Specific tracer uptake via the neural NET system is the most important basic characteristic for imaging presynaptic sympathetic innervation and norepinephrine handling (12,13). Desipramine is the most commonly used selective norepinephrine uptake inhibitor for the uptake-1 mechanism at the nerve terminal (14). In the present in vivo rabbit study, all tested tracers—18F-LMI1195, 11C-hydroxyephedrine, and 123I-MIBG—had a high affinity for neural uptake as demonstrated by the desipramine blockade. First of all, NET-mediated uptake of 18F-LMI1195 by in vitro cell uptake studies using human neuroblastoma cells demonstrated kinetic values comparable to norepinephrine (4). In a subsequent study, 18F-LMI1195 was tested using an isolated perfused rabbit heart system to avoid the effect of systemic recirculation of tracer and tracer metabolites (15). First-pass 18F-LMI1195 extraction was measured as 44% and 28% using a flow value of 2 and 4 mL/min/g, respectively. Desipramine added into the perfusion buffer markedly reduced the extraction to 4% at 4 mL/min/g. Furthermore, in vivo PET studies have confirmed the high contrast of 18F-LMI1195 cardiac uptake in rabbits, monkeys, and humans (4,5). Notably, the specific neural transport observed in these species is not the same as seen in small rodents (16,17). Desipramine treatment did not change the cardiac 18F-LMI1195 uptake in rat hearts, whereas phenoxybenzamine, a potent norepinephrine nonselective NET inhibitor (18), significantly reduced the tracer uptake. Therefore, 18F-LMI1195 transport via the extraneural uptake-2 mechanism was suggested and is consistent with transport considerably mediated by uptake-2 in the small rodent hearts. On the other hand, 11C-hydroxyephedrine demonstrated sensitivity for the desipramine treatment even in the in vivo rat heart, whereas 123I-MIBG uptake was not affected in the same way as 18F-LMI1195 uptake (19). Consistent with these findings, DeGrado et al. also demonstrated the high affinity of 11C-hydroxyephedrine to uptake-1 using isolated perfused rat hearts (20). The precise mechanism of these species- and tracer-dependent uptake variations needs to be further investigated. It is known that there are significant species variations in physiologic norepinephrine handling in the heart (21). These include the level of extraneural uptake-2 mechanism, as well as variations in the characteristics of the NET itself.
Although all available presynaptic sympathetic nerve norepinephrine analog tracers are designed to be initially transported into nerve terminals to visualize the nervous system, significant differences in intraneural kinetics have been reported among these tracers (22,23). Understanding this subcellular tracer handling is essential for fundamental interpretation of the imaging results. To investigate the retention mechanism, we tested 3 tracers with a neural NET inhibitor chase treatment; therefore, desipramine was added only at the washout phase just after initial tracer uptake.
Increased tracer washout from the heart after a desipramine chase was observed for 11C-hydroxyephedrine. This result is consistent with earlier studies performed with isolated perfused rat hearts and in vivo rat imaging (20,24). These findings indicate that 11C-hydroxyephedrine cardiac retention is maintained by continuous reuptake via NET and release (diffusion) from the nerve terminals. Norepinephrine is stored in the synaptic vesicles at nerve terminals, and when a firing impulse arrives at the synaptic ending, the sympathetic nerve neurotransmitter norepinephrine is released into the synaptic cleft via vesicular exocytosis. 11C-hydroxyephedrine cardiac retention reflects NET reuptake activity, while being less influenced by this vesicular turnover.
In contrast, 18F-LMI1195, similar to 123I-MIBG, demonstrated stable tracer retention resistant to the NET inhibitor chase treatment. 18F-LMI1195 and 123I-MIBG share a benzylguanidine structure (Fig. 1), possibly explaining the similarity of response to the desipramine chase. Consistent with these findings, isolated perfused rabbit heart experiments demonstrated no effect from the addition of desipramine to the perfusion buffer after the tracer uptake phase (15). This indicates stable tracer retention independent of NET activity. Furthermore, electrical field stimulation enhanced cardiac 18F-LMI1195 washout significantly in the isolated rabbit heart study (15). The stimulation is known to evoke norepinephrine overflow via vesicular release into the synaptic cleft at the nerve terminals (25). Therefore, the enhanced 18F-LMI1195 washout by the electrical provocation suggests the possibility of 18F-LMI1195 storage in the synaptic vesicle.
Integrating the available information, 18F-LMI1195 is most likely to be readily transported and stored into synaptic vesicles after being taken up into the nerve terminals, mimicking the norepinephrine vesicular turnover pathway. Thereby, dynamic 18F-LMI1195 PET to measure tracer washout kinetics would provide parameters not only for uptake-1 function but also for sympathetic afferent activity correlated with norepinephrine spillover from the heart. Although further confirmatory experiments are required, such as reserpine (vesicular transport inhibitor) blocking studies to exclude the possibility of nonspecific localization in intraneuronal membranes, our study demonstrates the potential of 18F-LMI1195 for monitoring the handling of norepinephrine at the sympathetic nerve terminals, including vesicular turnover at the myocardium.
CONCLUSION
18F-LMI1195, a novel 18F-labeled benzylguanidine analog PET tracer, demonstrates specific uptake in the heart as assessed by in vivo PET imaging in rabbits. Stable retention under a desipramine chase indicates promising properties as a new class of PET tracer for visualizing the cardiac nervous system, mimicking physiologic norepinephrine turnover at nerve terminals.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This work was supported by the Competence Network of Heart Failure funded by the Integrated Research and Treatment Center (IFB) of the Federal Ministry of Education and Research (BMBF) and German Research Council (DFG grant HI 1789/2-1). No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Jul. 16, 2015.
- © 2015 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication March 30, 2015.
- Accepted for publication July 7, 2015.