Abstract
One aim of the current study was to determine normalized dose data for maternal radiosensitive organs and embryo/fetus from 256-slice CT pulmonary angiography (CTPA) performed on pregnant patients suspected of having pulmonary embolism. A second aim was to provide reliable maternal and fetal doses and associated radiation cancer risk estimates from 256-slice CTPA and lung perfusion scintigraphy (LPS) for comparison. Methods: Mathematic anthropomorphic phantoms were generated to simulate the average woman at early pregnancy and at the third, sixth, and ninth months of gestation. In each phantom, 0–3 additional 1.5-cm-thick fat tissue layers were added to derive 4 phantoms representing pregnant women with different body sizes. Monte Carlo methods were used to simulate low-dose 256-slice CTPA exposures on each of the 16 generated phantoms. Normalized organ and embryo/fetal dose data were derived for exposures at 80, 100, and 120 kV. Maternal effective dose and embryo/fetal dose from 256-slice CTPA and associated lifetime attributable risks of radiation cancer were determined for different body sizes and gestational stages and compared with corresponding data from LPS. Results: For an average-sized pregnant patient at the first trimester, the 256-slice CTPA exposure resulted in a maternal effective dose of 1 mSv and an embryo/fetal dose of 0.05 mGy. However, maternal effective dose considerably increased with body size, whereas embryo/fetal dose increased with both body size and gestational stage. Compared with LPS, low-dose CTPA to an average-sized pregnant patient resulted in a 30% higher maternal effective dose but a 3.4–6 times lower embryo/fetal dose. Nevertheless, LPS was associated with less aggregated radiation risk for an average-sized pregnant patient, with the difference from CTPA being increased further for larger patients. Conclusion: Compared with CTPA performed with a modern wide-area CT scanner, LPS remains comparatively more dose-efficient.
Despite being a rather rare complication, occurring in only 1 in 1,000 pregnancies, pulmonary embolism (PE) is the leading nonobstetric cause of maternal death. About 1 in 100 pregnant patients diagnosed with PE die from this complication (1). Evaluation of suspected PE during pregnancy is quite challenging since clinical symptoms may be nonspecific. Despite having been proposed as a first-line imaging modality, lower limb ultrasonography has much lower sensitivity than chest radiography, lung perfusion scintigraphy (LPS), and CT pulmonary angiography (CTPA) (2). However, those 3 imaging approaches use ionizing radiation and, therefore, are associated with a certain radiation dose burden for both the expectant mother and the embryo/fetus. The significance of early diagnosis and treatment of PE during pregnancy and concerns about radiation from the available imaging tests have enhanced debate on which diagnostic strategy to follow (2).
To provide guidance for clinicians, the American Thoracic Society recently published evidence-based guidelines and recommendations for the management of pregnant patients suspected of having PE (3). In the document, chest radiography is recommended as the first radiation-associated imaging procedure. If the results are normal, LPS rather than CTPA is preferred as the next imaging test, although multidetector CTPA is currently considered either of equal diagnostic performance (4) or even the reference standard for the diagnosis of PE in nonpregnant patients (5). If the results of chest radiography are abnormal, CTPA is recommended as the next step of investigation, but this is a weak recommendation based on low-quality evidence (3). The rationale behind the above recommendations has been simple: CTPA delivers a significantly higher dose to the expectant mother and slightly less absorbed radiation dose to the fetus than LPS. However, the comparatively low radiation doses delivered to the embryo/fetus from either test, that is, much lower than 1 mGy, are not associated with a measurably increased risk for radiation-induced defects. In contrast, compared with lung scintigraphy, CTPA delivers a much higher radiation dose to the most radiosensitive organs of the expectant mother, that is, the breast and the lung, and therefore may be associated with an increased potential risk for radiation-induced malignancies.
The recent American Thoracic Society guidelines (3) are based on dosimetric data associated with up to 64-slice CT scanners, which were the state of the art about a decade ago. CT technology, however, has evolved rapidly over the last few years, providing 128–320 imaged slices per rotation and novel tools for reducing dose to the patient (6). Patient dose from standard examinations performed on modern wide-area-detector CT scanners is expected to be comparatively lower (7). Besides, radiation dose to the expectant mother from CTPA performed during pregnancy has been reported previously and compared with the corresponding value from LPS in terms of effective dose determined through the dose–length product and a non–sex-specific factor to convert dose–length product to effective dose (8,9). However, the use of an effective dose derived from dose–length product for quantifying radiogenic cancer risk has been strongly criticized (10–13). Currently, the use of the absorbed doses to radiosensitive organs and corresponding sex-, age- and organ-specific radiogenic cancer risk factors proposed by the Biologic Effects of Ionizing Radiation (BEIR) Committee constitute the best means to evaluate radiation risk from CT exposures (13,14).
The current study was motivated by the absence of data on radiation dose burden and associated radiogenic risks from CTPA performed on pregnant women with modern, wide-area-detector CT scanners. Given the widely accepted high diagnostic value of CTPA, the rationale of recommending LPS rather than CTPA in the management of pregnant patients might be cancelled if radiation risks for both the expectant mother and the embryo/fetus from modern CTPA studies prove to be equal to or lower than corresponding radiation risks from lung scintigraphy.
One aim of the present study was to determine normalized data on absorbed doses to radiosensitive organs and the developing embryo/fetus from 256-slice CTPA studies performed on pregnant patients of different sizes and gestational stages suspected of having PE. A second aim was to provide reliable data on maternal and fetal dose burden and associated radiogenic cancer risk estimates for pregnant patients who undergo 256-slice CTPA, in comparison with LPS.
MATERIALS AND METHODS
CT Scanner and CTPA Protocol
A modern 256-slice scanner (Brilliance iCT; Philips Healthcare) was used in the current study. Equipped with alternating focal-spot technology, this scanner uses a 128 × 0.625 mm detector array that allows for 80 mm of z-coverage per rotation. This scanner may also use several advanced tools to reduce the exposure of the patient, such as automatic tube current modulation, adaptive section collimation, examination-specific wedge filters, and iterative reconstruction.
CTPA examinations are routinely performed at end-inspiration and cover the region from the lung apices to below the diaphragm. The typical exposure settings for adult nonpregnant patients suspected of having PE are a tube voltage–load of 120 kV–200 mAs, collimation of 128 × 0.625 mm, pitch of 1, rotation time of 0.5 s, adaptive-section collimation, and large wedge-filter. For pregnant patients, low-dose techniques are commonly applied. In this study, the availability of iterative reconstruction allowed for low tube voltage–load combinations depending on the body size of the patient at the time of conception according to the scheme shown in Table 1, which conforms to a recently proposed scheme for exposure parameter selection for low-dose 256-slice CTPA (15).
Tube Voltage–Load Selection vs. Body Size for Low-Dose CTPA Studies on Pregnant Patients
Simulation of 256-Slice CTPA Examinations
CTPA exposures were simulated on mathematic anthropomorphic phantoms using Monte Carlo methods. Implemented on a personal computer–based software platform, the Monte-Carlo N-particle transport code was used. The exposure geometry and the spectrum of the CT beam were fed to the software through a generated input file. X-ray spectra at 80, 100, and 120 kV were produced using the method of Boone and Seibert (16) for the specific total filter of the scanner.
Mathematic anthropomorphic phantoms were generated by the Bodybuilder software package, version 1.3 (White Rock Science), to simulate the average woman at the time of conception and at the third, sixth, and ninth gestational months. To study the effect of body size on maternal organ doses and embryo/fetal dose, 1–3 fat-tissue layers of 1.5-cm thickness each were uniformly added to the body trunk of each phantom to correspond to a specific gestational stage. Thus, 4 phantoms of different body size were generated for each gestational stage. The height, weight, and chest circumference, along with the associated body mass index (BMI), of generated mathematic anthropomorphic phantoms are shown in Table 2. All female radiosensitive organs (17) were represented in the generated mathematic phantoms. The embryo dose during the first weeks of gestation was assumed to equal the uterus dose of the phantom at conception. Fetal dose at the end of the first, second, and third trimesters was determined as the mean dose delivered to the whole fetus.
Somatometric Characteristics of Generated Anthropomorphic Phantoms Simulating Pregnant Individuals
Maternal Organ and Embryo/Fetal Doses from 256-Slice CTPA
After each CTPA exposure simulation, the derived absorbed radiation doses to maternal organs and embryo/fetus were normalized to the free-in-air measurement of the CT dose index at isocenter (CTDIfree-in-air) for the tube voltage used. Normalized data were derived for 4 body sizes at conception, 4 gestational stages, and 3 tube voltages. The absorbed doses to the radiosensitive organs and the embryo/fetus of a specific pregnant patient after a specific CTPA exposure were estimated by multiplying the normalized data for that specific body size, gestational stage, and tube voltage by the measured value of CTDIfree-in-air for the tube voltage–load used for the prescribed patient exposure.
The normalized maternal effective dose was determined using the following formula and data provided in the latest recommendations of the International Commission on Radiological Protection (17):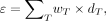 Eq.1where wT and dT are the weighting factors proposed by the International Commission on Radiological Protection and the normalized doses for the radiosensitive organs/tissues T, respectively.
Eq.1where wT and dT are the weighting factors proposed by the International Commission on Radiological Protection and the normalized doses for the radiosensitive organs/tissues T, respectively.
Maternal Organ and Embryo/Fetal Doses from LPS
The radiopharmaceutical exclusively used for LPS is 99mTc-macroaggregated albumin, and the usual administered activity in adults is 40–150 MBq (18–20). Given the low incidence of lung comorbidity in young patients and the need to minimize radiation risks for both the expectant mother and the embryo/fetus, in many centers planar LPS is performed on pregnant patients with a reduced administered activity and without the additional step of ventilation imaging used in nonpregnant patients. In the current study, the typical administered dose for LPS in pregnant patients was considered to be 60 MBq (18–20).
The absorbed doses to maternal radiosensitive organs, as well as the effective dose, from the typical LPS were estimated using organ dose data normalized to megabecquerel of administered activity as published by the International Commission on Radiological Protection (21). The dose to the embryo/fetus was estimated using previously published conversion factors of 0.0028, 0.0040, 0.0050, and 0.0040 mGy/MBq of administered activity for early, 3-, 6-, and 9-mo pregnancy, respectively (20).
Estimation of Maternal and Embryo/Fetal Radiogenic Cancer Risks from CTPA and LPS
The total projected risk for radiation-induced cancer, or life-attributable risk (LAR), was estimated for low-dose 256-slice CTPA and LPS in pregnant patients of varying BMI at conception (19.7, 22.9, 26.3, and 30.1 kg/m2) and age (20, 30, and 40 y). First, the organ-specific LARs associated with CTPA and LPS were estimated using the derived organ dose data for the specific patient BMI and the corresponding radiation cancer risk factors for the patient age provided by the BEIR VII Committee (14). Then, the total cancer risk for a specific patient undergoing either CTPA or LPS was calculated as the sum of all corresponding organ-specific LARs.
The risk of childhood cancer for an embryo/fetus exposed after CTPA or LPS was estimated by multiplying the corresponding dose by a risk factor, which has been proposed to be 0.06% per 10 mGy (22).
RESULTS
Normalized data derived for pregnant patients of varying body size and gestational stage undergoing low-dose 256-slice CTPA are shown in Tables 3, 4, and 5 for 120, 100, and 80 kV, respectively. These data may be used to estimate absorbed doses to maternal radiosensitive organs or embryo/fetus for any pregnant patient undergoing 256-slice CTPA at any gestational stage given the free-in-air measurement of CTDI for the tube voltage–load values used. The organs receiving the highest doses from a CTPA exposure were breast, lung, and heart. Table 6 presents maternal organ, embryo/fetal, and effective doses estimated for pregnant patients of varying BMI at conception undergoing low-dose 256-slice CTPA at the end of the first trimester.
Normalized (to CTDIfree-in-air) Maternal Organ and Embryo/Fetal Dose Data for Pregnant Patients Subjected to CTPA Exposure at 120 kV
Normalized (to CTDIfree-in-air) Maternal Organ and Embryo/Fetal Dose Data for Pregnant Patients Subjected to CTPA Exposure at 100 kV
Normalized (to CTDIfree-in-air) Maternal Organ and Embryo/Fetal Dose Data for Pregnant Patients Subjected to CTPA Exposure at 80 kV
Maternal Organ and Embryo/Fetal Doses (mGy) of Pregnant Patients Undergoing 256-Slice CTPA at End of First Trimester
The effective dose to pregnant patients undergoing low-dose CTPA was strongly dependent on patient body size, since the effective dose more than tripled when BMI increased from 19.7 to 30.1 kg/m2, whereas dependence on the gestational stage was minimal, since maximum difference was less than 7%. The effective dose to pregnant patients undergoing low-dose LPS was 0.75 mSv. The effective dose to pregnant patients of varying body size and gestational stage undergoing low-dose CTPA are shown in Figure 1, together with the corresponding value from low-dose LPS. The effective dose to pregnant patients from CTPA was considerably higher than that from LPS irrespective of patient body size and gestational stage.
Effective dose to pregnant patients of varying body size and gestational stage undergoing low-dose CTPA. Corresponding value for low-dose LPS is shown for comparison (dashed line).
Embryo/fetal dose in pregnant patients undergoing low-dose CTPA strongly increased with increasing BMI, and the dependence on gestational stage was considerable: embryo/fetal dose increased by 25%–80% as the pregnancy progressed. That finding may be attributed to the fact that as the embryo/fetus grows, the embryonic tissues approach the primarily exposed body region, thus receiving higher amounts of scattered radiation. The embryo dose from low-dose LPS was 0.17, 0.24, 0.30, and 0.24 mGy at early pregnancy and at the first, second, and third trimesters, respectively. The embryo/fetal dose from low-dose CTPA performed on pregnant patients of varying body size and gestational stage is shown in Figure 2, together with the corresponding values from LPS. Embryo/fetal dose from low-dose 256-slice CTPA was lower than the corresponding dose from low-dose LPS, with the exception of pregnant patients with a BMI greater than 30 kg/m2 at the end of the third trimester of pregnancy.
Embryo/fetal dose from low-dose CTPA performed on pregnant patients of varying body size and gestational stage. Corresponding data for low-dose LPS are shown for comparison.
The total LAR of cancer in pregnant patients of varying age and BMI at conception after low-dose 256-slice CTPA is shown in Figure 3, together with corresponding LARs associated with LPS. The 256-slice CTPA–associated LAR of cancer was higher than the corresponding LPS-associated LAR in all cases. The difference between CTPA- and LPS-associated LARs increased with pregnant patient BMI, decreased with patient age, and remained essentially unchanged during all stages of pregnancy. This difference was 6–23, 2–14, and 0–14 per 100,000 average-sized pregnant patients at the age of 20, 30, and 40 y, respectively. The risks of childhood cancer for the embryo/fetus exposed to low-dose CTPA are shown in Figure 4, together with the corresponding risks associated with LPS. The 256-slice CTPA–associated risk for childhood cancer was lower than the corresponding LPS-associated risk, with the exception of large pregnant patients (BMI > 30 kg/m2) at the third trimester. The difference between CTPA- and LPS-associated risks of childhood cancer decreased with pregnant patient BMI. This difference was below 1.5 per 100,000 for all stages of pregnancy and maternal body sizes.
Maternal total LAR of cancer from low-dose 256-slice CTPA in pregnant patients of varying age and BMI at conception for early pregnancy (A) and for first (B), second (C), and third (D) trimesters of pregnancy. Corresponding LARs from low-dose LPS are shown for comparison.
Risks of childhood cancer for embryo/fetus exposed because of low-dose CTPA performed on expectant mother of varying BMI at conception for early pregnancy (A) and for first (B), second (C), and third (D) trimesters of pregnancy. Corresponding risks from low-dose LPS are shown for comparison.
DISCUSSION
This study estimated the maternal and embryo/fetal radiation burden and associated risks of radiation-induced cancer from low-dose 256-slice CTPA performed on pregnant patients suspected of having PE and compared these values with the corresponding values from low-dose LPS, which is the alternative diagnostic method. This work revealed that modern wide-area CT scanners allow for CTPA studies resulting in a maternal effective dose of as low as 1 mSv and an embryo/fetal dose of 0.05 mGy for average-sized (BMI, 19 kg/m2) pregnant patients suspected of having PE. However, both the resulting maternal effective dose and the embryo/fetal dose from CTPA considerably increased with patient BMI at conception, for example, up to 340% and 400%, respectively, for patients with a BMI of 30 kg/m2. As gestation progressed, embryo/fetal dose increased by 20%–80% whereas maternal effective dose remained essentially unaffected. Besides, the maternal effective dose and embryo/fetal dose from LPS were 0.75 mSv and 0.17–0.30 mGy, respectively. Compared with LPS, low-dose CTPA in average-sized pregnant patients resulted in a 30% higher maternal effective dose but a 3.4–6 times lower embryo/fetal dose. Nevertheless, such a comparison is inconclusive regarding the dilemma of which test to prefer in the case of a pregnant patient suspected of having PE. Assuming similar diagnostic performance for CTPA and LPS in PE, the main criterion to perform one test over the other should be the level of associated radiation risk rather than the effective dose. Other factors to consider include availability, local expertise, contrast toxicity, cost, and the potentially increased number of technically unsatisfactory CTPAs. Compared with LPS, low-dose 256-slice CTPA resulted in a higher total maternal LAR of cancer but much lower embryo/fetal risk for childhood cancer. However, embryo/fetal risk for childhood cancer after either CTPA or LPS was more than 1 order of magnitude lower than the corresponding maternal radiogenic risks of cancer. Consequently, LPS is associated with less aggregated radiation risk for an average-sized pregnant patient and her fetus whereas the difference from CTPA is broadened for patients with higher BMIs. In addition, this difference increases further as pregnancy progresses since the growing fetus absorbs more CTPA-related scattered radiation. Current results indicate that LPS is still comparatively more dose-efficient and should definitely remain the preferable next imaging step in pregnant patients suspected of PE who have normal chest radiography findings and require further investigation. Moreover, the current results enhance the rationale reported by Freeman (23) for not abandoning LPS in the evaluation of patients with suspected PE. However, associated maternal radiation cancer risks from both low-dose 256-slice CTPA and optimized LPS are very low compared with the corresponding nominal risks for maternal cancer induction. Indeed, the lifetime risk of cancer has been recently reported to be 38.44%, 38.29%, and 37.67%, for 20-, 30- and 40-y-old women, respectively (24). Therefore, if an average-sized pregnant patient suspected of having PE is undergoing low-dose CTPA at the age of 20, 30, or 40 y, the total LAR of radiogenic cancer is added to the lifetime risk, which is marginally increased by a factor of 1.0007, 1.0004, and 1.0003, respectively. Consequently, the recommendation to proceed with LPS rather than CTPA after normal chest radiography results should be followed if both imaging modalities are available. If, however, LPS is not possible, avoidance of CTPA in pregnant patients with suspected PE cannot be justified solely on the grounds of the associated radiogenic cancer risks.
The mean maternal effective dose from standard CTPA studies with 64-slice CT scanners has been reported to be 7.3 mSv (9) whereas the corresponding value for low-dose protocols has been reported to be 1.8 mSv (25). Recently, Viteri-Ramirez et al. (8) reported a mean effective dose of 1.1 mSv from low-dose CTPA studies performed on a state-of-the-art dual-source CT scanner—a value that closely agrees with the current results. Apparently, the patient radiation burden from CTPA may be considerably reduced if a modern CT scanner is used. Besides, the embryo/fetal dose from CTPA studies has been reported to be 0.06–0.23 mGy during the third trimester of pregnancy (26), which agrees with our findings of 0.05–0.28 mGy. Discordantly, Hurwittz et al. (27) reported an embryo/fetal dose of 0.24–0.66 mGy from CTPA studies performed on pregnant patients with suspected PE during the first trimester. The considerable difference between these results and our data for the first trimester, that is, 0.05–0.16 mGy, may be attributed to the considerably higher exposure settings used in the study by Hurwitz et al., that is, 140 kV–300 mAs.
The data presented in Tables 3–5 may be used to estimate organ doses, effective dose, and dose to embryo/fetus from 256-slice CTPA performed on pregnant patients of any size and gestational stage, even before the examination. If the CT scanner available for CTPA differs from ours, embryo/fetal and effective doses may be estimated using the following formula (28): Eq. 2where D, Dx are the doses (organ, embryo/fetus or effective) and (CTDIw/CTDIfree-in-air), (CTDIw/CTDIfree-in-air)X are the ratios of the weighted to free-in-air CTDI values for the 256-slice CT scanner considered here and scanner X, respectively, for the specific voltage and amperage used during the CTPA exposure. The uncertainty associated with such an estimation has been reported to be less than 10% (28).
Eq. 2where D, Dx are the doses (organ, embryo/fetus or effective) and (CTDIw/CTDIfree-in-air), (CTDIw/CTDIfree-in-air)X are the ratios of the weighted to free-in-air CTDI values for the 256-slice CT scanner considered here and scanner X, respectively, for the specific voltage and amperage used during the CTPA exposure. The uncertainty associated with such an estimation has been reported to be less than 10% (28).
The dose data presented in Tables 3–5 were derived for a 256-slice CTPA study of specific scanning length along the z-axis. Being an operator-defined parameter, total imaged volume may vary between patients, and uncertainty is therefore introduced to dose estimations using these data. However, given that CTPA in pregnant patients should be performed cautiously so that all exposure parameters are optimized, the scanning length should be set to the minimum required and, consequently, the imaged volume is not expected to differ considerably between CTPA examinations. Differences between the anthropomorphic phantoms used for CTPA simulations and the phantoms used to derive the organ-dose-per-administered-activity factors may introduce uncertainties in the presented dosimetric comparisons; however, such uncertainties are expected to be minor given that both phantoms represent average individuals. Also, maternal and embryo/fetal doses were estimated using organ-dose factors not adjusted for patient BMI. This, however, was a one-way approach since no relevant data are available in the literature. Another source of uncertainty in current estimates of radiation risks originates from the absence of rigid data for the precise quantification of such risks after exposures to low-level absorbed radiation doses. The risk factors we have used were derived by the BEIR Committee of the National Research Council by extrapolating linearly below the existing range of accurate observations for high absorbed radiation doses to humans. In other words, the BEIR Committee accepts the so-called linear nonthreshold model for the prediction of theoretic radiogenic cancer incidence after exposure to low levels of radiation. Although the existing data on the effects of low-level radiation doses are inconclusive, most investigators accept the validity of the linear no-threshold model since, presently, no alternative dose–response relationship for the carcinogenic effect of low-level radiation appears to be more plausible.
CONCLUSION
Although the maternal and embryo/fetal absorbed radiation dose after CTPA may be considerably reduced when modern wide-area-detector CT scanners are used, LPS remains comparatively more dose-efficient. The data presented here may be used to assess both maternal and embryo/fetal radiation doses and associated cancer risks from any CTPA procedure performed on a pregnant patient suspected of having PE.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was supported by the Greek Ministry of Education and Religious Affairs, General Secretariat for Research and Technology, operational program “Education and Lifelong Learning,” ARISTEIA (research project CONCERT). No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online May 29, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFFERENCES
- Received for publication January 24, 2014.
- Accepted for publication April 25, 2014.











