Abstract
The galactose analog 2-18F-fluoro-2-deoxy-d-galactose (18F-FDGal) is a suitable PET tracer for measuring hepatic galactokinase capacity in vivo, which provides estimates of hepatic metabolic function. As a result of a higher affinity of galactokinase toward galactose, the lumped constant (LC) for 18F-FDGal was 0.13 in healthy subjects. The aim of the present study was to test the hypothesis of a significantly different LC for 18F-FDGal in patients with parenchymal liver disease. Methods: Nine patients with liver cirrhosis were studied in connection with a previous study with determination of hepatic intrinsic clearance of 18F-FDGal (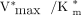 ). The present study determined the hepatic removal kinetics of galactose, including hepatic intrinsic clearance of galactose (
). The present study determined the hepatic removal kinetics of galactose, including hepatic intrinsic clearance of galactose ( /
/ ) from measurements of hepatic blood flow and arterial and liver vein blood galactose concentrations at increasing galactose infusions. LC for 18F-FDGal was calculated as (
) from measurements of hepatic blood flow and arterial and liver vein blood galactose concentrations at increasing galactose infusions. LC for 18F-FDGal was calculated as ( )/(
)/(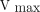 /
/ ). On a second day, a dynamic 18F-FDGal PET study with simultaneous infusion of galactose (mean arterial galactose concentration, 6.1 mmol/L of blood) and blood samples from a radial artery was performed, with determination of hepatic systemic clearance of 18F-FDGal (
). On a second day, a dynamic 18F-FDGal PET study with simultaneous infusion of galactose (mean arterial galactose concentration, 6.1 mmol/L of blood) and blood samples from a radial artery was performed, with determination of hepatic systemic clearance of 18F-FDGal ( ) from linear analysis of data (Gjedde–Patlak method). The maximum hepatic removal rate of galactose was estimated from 18F-FDGal PET data (
) from linear analysis of data (Gjedde–Patlak method). The maximum hepatic removal rate of galactose was estimated from 18F-FDGal PET data ( ) using the estimated LC. Results: The mean hepatic
) using the estimated LC. Results: The mean hepatic  of galactose was 1.18 mmol/min, the mean
of galactose was 1.18 mmol/min, the mean  was 0.91 mmol/L of blood, and the mean
was 0.91 mmol/L of blood, and the mean  /
/ was 1.18 L of blood/min. When compared with values from healthy subjects,
was 1.18 L of blood/min. When compared with values from healthy subjects,  did not differ (P = 0.77), whereas both
did not differ (P = 0.77), whereas both  and
and  /
/ were significantly lower in patients (both P < 0.01). Mean LC for 18F-FDGal was 0.24, which was significantly higher than the mean LC of 0.13 in healthy subjects (P < 0.0001). Mean
were significantly lower in patients (both P < 0.01). Mean LC for 18F-FDGal was 0.24, which was significantly higher than the mean LC of 0.13 in healthy subjects (P < 0.0001). Mean  determined from the PET study was 0.019 L of blood/min/L of liver tissue, which was not significantly different from that in healthy subjects (P = 0.85). Mean hepatic
determined from the PET study was 0.019 L of blood/min/L of liver tissue, which was not significantly different from that in healthy subjects (P = 0.85). Mean hepatic  was 0.57 mmol/min/L of liver tissue, which was significantly lower than the value in healthy subjects (1.41 mmol/min/L of liver tissue (P < 0.0001)). Conclusion: Disease may change the LC for a PET tracer, and this study demonstrated the importance of using the correct LC.
was 0.57 mmol/min/L of liver tissue, which was significantly lower than the value in healthy subjects (1.41 mmol/min/L of liver tissue (P < 0.0001)). Conclusion: Disease may change the LC for a PET tracer, and this study demonstrated the importance of using the correct LC.
PET is an excellent noninvasive tool for in vivo studies of metabolic processes. The PET tracers used may be natural substrates radiolabeled with positron-emitting isotopes such as 11C. However, analogs of natural substrates are commonly used, a well-known example being the glucose analog 18F-FDG used for studies of glucose metabolism. Using an analog tracer is advantageous when its metabolism is simpler, such as the metabolism of 18F-FDG, which, unlike that of glucose, essentially stops after 6-phosphorylation. Fewer kinetic parameters are thus required in the kinetic model fitted to the dynamic PET data. An important disadvantage of using an analog tracer is that it may differ from the natural substrate in its affinities for blood-to-cell transporters and intracellular enzymes responsible for the metabolism. The result of this difference is that the measured tracer kinetics may not be equal to the kinetics of the tracee. As a correction, the lumped constant (LC) has been introduced—a proportionality constant used to infer the metabolic rate of the tracee from the measured metabolic rate of the tracer. The LC is defined as follows: Eq. 1that is, the ratio between the intrinsic clearances of tracer (asterisk) and the tracee (1,2). The partition coefficients across the plasma membrane for the 2 substrates are included in estimates of the intrinsic clearances when determined in vivo (2).
Eq. 1that is, the ratio between the intrinsic clearances of tracer (asterisk) and the tracee (1,2). The partition coefficients across the plasma membrane for the 2 substrates are included in estimates of the intrinsic clearances when determined in vivo (2).
The purpose of the present study was to determine the LC for the radiolabeled galactose analog 2-18F-fluoro-2-deoxy-d-galactose (18F-FDGal) in patients with liver disease. Both galactose and 18F-FDGal are substrates for galactokinase, a highly substrate-specific enzyme found almost exclusively in hepatocytes and responsible for 1-phosphorylation of both substrates. In healthy human subjects, the mean LC for 18F-FDGal was found to be 0.13 and used to calculate the maximum hepatic removal rate of galactose (Vmax) from dynamic 18F-FDGal PET data obtained during a constant infusion of galactose (3). Hepatic Vmax of galactose, which is commonly estimated by the so-called galactose elimination capacity test, is interesting because it yields a measure of overall metabolic liver function and provides important prognostic information for patients with acute and chronic liver disease (4–6). On the basis of unpublished experiences in cirrhotic patients, we have reasons to believe that parenchymal liver disease may significantly affect the LC for 18F-FDGal, and in the present study we tested this hypothesis by determining the LC for 18F-FDGal in patients with liver cirrhosis. If liver disease affects the LC, knowledge of the correct LC is important for correct calculation of the maximum hepatic removal rate of galactose from 18F-FDGal PET data (i.e.,  ).
).
MATERIALS AND METHODS
Hepatic removal kinetics of galactose in vivo, including hepatic Vmax and Km, were determined in 9 patients with liver cirrhosis. The investigations were performed in connection with a previously published study in which the hepatic removal kinetics of 18F-FDGal, including the ratio  , were determined (7). This experimental setup allowed us to calculate individual values of LC according to Equation 1.
, were determined (7). This experimental setup allowed us to calculate individual values of LC according to Equation 1.
For in vivo estimation of the maximum hepatic removal rate of galactose in liver parenchyma from 18F-FDGal PET ( , mmol/L of liver tissue/min), the patients underwent a dynamic 18F-FDGal PET/CT study within 2–3 wk with a simultaneous infusion of galactose and blood sampling from a radial artery.
, mmol/L of liver tissue/min), the patients underwent a dynamic 18F-FDGal PET/CT study within 2–3 wk with a simultaneous infusion of galactose and blood sampling from a radial artery.
No data from the present study have been published before.
The study was approved by the Central Denmark Region Committees on Biomedical Research Ethics and conducted in accordance with the 1975 Declaration of Helsinki. Informed written consent was obtained from all patients. No complications to the procedures were observed.
Hepatic Removal Kinetics of Galactose and LC for 18F-FDGal
The hepatic removal rate of galactose was measured at 6 different steady-state blood concentrations of galactose ranging from low to high, that is, from approximate first-order to near-saturated kinetics. The steady-state blood concentrations were achieved by intravenous infusions of galactose (Aarhus University Hospital Pharmacy) at 6 increasing doses (0.70–2.90 mmol/min). Each of the 6 measurement periods was preceded by a 15- to 35-min equilibration period after adjusting the infusion dose. The last 3 periods were furthermore initiated by a priming dose of 10–20 mmol of galactose. At each blood concentration level, 4 pairs of blood samples were collected from a radial artery and a liver vein for determination of blood galactose concentrations (8). Good approximation to constant galactose concentrations was reached in all infusion rates, with no systematic deviation, and the mean galactose concentrations in the artery (A, mmol/L of blood) and liver vein (V, mmol/L of blood) from each concentration level were used to calculate the maximum hepatic removal rate (Vmax, mmol/min) and the Michaelis constant (Km, mmol/L of blood) for galactose according to the sinusoidal perfusion model of in vivo enzymatic elimination (9,10) by nonlinear regression of the Michaelis–Menten relationship to data: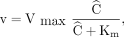 Eq. 2using the 6 sets of v and Ĉ, where v is the removal rate of galactose (mmol/min) calculated as F(A − V) at a given Ĉ (mmol/L of blood) calculated as (A − V)/ln(A/V). F is the hepatic blood flow (L of blood/min) determined from indocyanine green infusion and Fick’s principle (3), and Ĉ accounts for the decreasing blood concentration of galactose along the flow direction in the sinusoids in vivo (9,10).
Eq. 2using the 6 sets of v and Ĉ, where v is the removal rate of galactose (mmol/min) calculated as F(A − V) at a given Ĉ (mmol/L of blood) calculated as (A − V)/ln(A/V). F is the hepatic blood flow (L of blood/min) determined from indocyanine green infusion and Fick’s principle (3), and Ĉ accounts for the decreasing blood concentration of galactose along the flow direction in the sinusoids in vivo (9,10).
The intrinsic clearance of galactose was calculated as Vmax/Km (L of blood/min), and the hepatic systemic clearance of galactose (Ksyst; L of blood/min) was calculated as follows (11): Eq. 3 The ratios of Ksyst to F and Ksyst to Vmax/Km were used to determine whether first-order hepatic removal kinetics of galactose are predominantly flow- or enzyme-determined (11).
Eq. 3 The ratios of Ksyst to F and Ksyst to Vmax/Km were used to determine whether first-order hepatic removal kinetics of galactose are predominantly flow- or enzyme-determined (11).
The LC for 18F-FDGal was calculated according to Equation 1, that is, as the ratio between the hepatic intrinsic clearance of 18F-FDGal (from 7) and the hepatic intrinsic clearance of galactose determined in the present study.
Hepatic Vmax of Galactose Measured by 18F-FDGal PET
For the PET/CT study, a catheter was placed in a radial artery for blood sampling and in a cubital vein in both arms for infusion of galactose and injection of 18F-FDGal. The galactose infusion (infusion rate, 2.7–5.1 mmol/min) was started 60–70 min before the PET recording with a priming dose of 30–45 mmol of galactose and continued until the end of the PET recording. The individual infusion dose was estimated from a galactose elimination capacity test (12,13) performed within 2 wk of the PET study.
The subject was placed supine in a 40-slice Biograph TruePoint PET/CT camera (Siemens AG), and a topogram of the abdomen was performed for optimal positioning of the liver within the 21.6-cm transaxial field of view of the PET camera. Next, a low-dose CT scan (50 effective mAs with CAREDose4D, 120 kV, pitch of 0.8, and slice thickness of 5 mm) was performed for definition of anatomic structures and attenuation correction of the PET data. Simultaneously with the start of a 60-min dynamic PET recording, a bolus of 100 MBq of 18F-FDGal (range, 96–104 MBq) in 10 mL of saline was administered intravenously, and the intravenous line flushed with 15 mL of saline. 18F-FDGal was produced as previously described, with a radiochemical purity of 98% ± 1% (14).
During the PET recording, blood samples (0.5 mL) were collected from the radial artery at 18 × 5, 6 × 10, 3 × 20, 3 × 60, 1 × 120, 1 × 240, 1 × 360, and 4 × 600 s for determination of 18F-FDGal blood concentrations. Radioactivity concentrations were measured in a well counter (Packard Instruments) and corrected for radioactive decay back to the start of the PET recording, yielding a blood time–activity curve (time–activity curveblood; kBq/L of blood vs. time in minutes). At time 0, 20, 40, and 60 min, arterial blood samples (0.5 mL) were collected for determination of blood concentration of galactose (8). In each individual, good approximation to a constant galactose concentration was achieved during the PET study, and the individual mean arterial galactose concentration (Ca, mmol/L of blood) was used for the calculation of  .
.
PET data were reconstructed using iterative processing and a time-frame structure of 18 × 5, 15 × 10, 4 × 30, 4 × 60, and 10 × 300 s (total, 60 min) and corrected for radioactive decay back to the start of the recording, yielding images of 128 × 128 × 47 voxels and a central spatial resolution of 6.7 mm (full width at half maximum); voxel size was 2.4 × 2.4 × 3.1 mm. Using the combined PET/CT images, a volume of interest was drawn in the liver tissue 1.5–2 cm from the edge of the liver and avoiding the central parts of the liver. For each volume of interest, the time–activity curve (time–activity curveliver; kBq/L of liver tissue vs. time in minutes) was generated.
The hepatic systemic clearance of 18F-FDGal per volume of liver tissue ( ; L blood/min/L of liver tissue) was calculated as the asymptote fitted to Gjedde–Patlak (15,16) representation of time–activity curveblood and time–activity curveliver using data from 6 to 20 min after the 18F-FDGal administration (quasi–steady-state metabolism) (3,7,17).
; L blood/min/L of liver tissue) was calculated as the asymptote fitted to Gjedde–Patlak (15,16) representation of time–activity curveblood and time–activity curveliver using data from 6 to 20 min after the 18F-FDGal administration (quasi–steady-state metabolism) (3,7,17).
The hepatic Vmax for galactose per volume of liver tissue ( ; mmol/min/L of liver tissue) was calculated as follows (3,17):
; mmol/min/L of liver tissue) was calculated as follows (3,17): Eq. 4The term (Km+Ĉ)/Ĉ corrects for the individual degree of saturation of the hepatic galactose elimination. At near-saturated kinetics, the difference between Ca and Ĉ becomes negligible, and Ca was accordingly used since V was not measured in this part of the experiment.
Eq. 4The term (Km+Ĉ)/Ĉ corrects for the individual degree of saturation of the hepatic galactose elimination. At near-saturated kinetics, the difference between Ca and Ĉ becomes negligible, and Ca was accordingly used since V was not measured in this part of the experiment.
Statistics
The estimation of Vmax and Km values included SEs of the estimates of v and Ĉ in the nonlinear regression of the implicit relationship between the variables given by Equation 2, using maximum likelihood estimation (10,18). Linear regression was used for the calculation of the hepatic systemic clearance of 18F-FDGal from PET data according to the Gjedde–Patlak method with maximum-likelihood estimation. The Student t test was used for comparison of mean values between cirrhotic patients and healthy subjects. A P value of less than 0.05 was considered to indicate a statistically significant difference.
RESULTS
Hepatic Removal Kinetics of Galactose and LC for 18F-FDGal
The mean hepatic Vmax of galactose was 1.18 mmol/min, and mean Km was 0.91 mmol/L of blood (Table 1). When compared with values from healthy subjects (3), Vmax was significantly lower (P < 0.01) whereas Km was not statistically significantly different (P = 0.77). Mean Vmax/Km was 1.18 L of blood/min (Table 1), which was significantly lower than that in healthy subjects (P < 0.001) (3). The estimates of Vmax and Km were highly correlated within each individual (mean correlation, 94%; range, 89%–97%), and the individual Vmax/Km ratios were accordingly estimated with high accuracy.
Patient Characteristics and Hepatic Removal Kinetics of Galactose and LC for 18F-FDGal in Patients with Liver Disease
Mean Ksyst was 0.80 L of blood/min, with a mean approximation to the hepatic blood flow of 73%, which was significantly lower (P = 0.001) than the approximation of Ksyst to the hepatic blood flow of 92% in healthy subjects (3). The mean approximation of Ksyst to Vmax/Km was 55%, which was significantly higher (P = 0.0001) than the approximation of 36% in healthy humans (3). Accordingly, although hepatic systemic clearance of galactose is predominantly flow-determined in patients with cirrhosis, the contribution from enzymatic capacity on the estimate is not negligible.
The LC for 18F-FDGal ranged from 0.18 to 0.29, with a weighted mean of 0.24 (Table 1), which was significantly higher (P < 0.0001) than the mean LC of 0.13 in healthy subjects (3).
There were no significant correlations between LC and Vmax, galactose elimination capacity, or  (all, P > 0.05).
(all, P > 0.05).
Hepatic Vmax of Galactose Measured by 18F-FDGal PET
Mean Ca during the PET recording was 6.1 mmol/L of blood (range, 4.7–7.5 mmol/L of blood). This yielded a mean approximation to saturated kinetics of 86% (range, 72%–92%). Mean  was 0.019 L of blood/min/L of liver tissue (Table 2), which was not significantly different from that in healthy subjects (P = 0.85) (3). Mean hepatic
was 0.019 L of blood/min/L of liver tissue (Table 2), which was not significantly different from that in healthy subjects (P = 0.85) (3). Mean hepatic  was 0.57 mmol/min/L of liver tissue (Table 2), which was significantly lower (P < 0.0001) than the value of 1.41 mmol/min/L of liver tissue in healthy subjects (3).
was 0.57 mmol/min/L of liver tissue (Table 2), which was significantly lower (P < 0.0001) than the value of 1.41 mmol/min/L of liver tissue in healthy subjects (3).
Hepatic Vmax for Galactose Determined from 18F-FDGal PET/CT
DISCUSSION
When quantitative PET studies of metabolic processes using a tracer analog are performed, it is important to know how disease may affect the LC for the tracer. The present study demonstrated how cirrhosis significantly increases the LC for the galactose analog 18F-FDGal in liver tissue when compared with previously published values of LC in healthy subjects determined using the same experimental setup (3).
In accordance with the fact that patients with cirrhosis have decreased metabolic liver function, Vmax was significantly lower than the mean value previously found in healthy subjects (Table 1) when measured directly from galactose infusions and blood samples from an artery and a liver vein. When hepatic galactose Vmax was calculated from the dynamic 18F-FDGal PET study with simultaneous infusion of galactose (i.e.,  ) and corrected for the individual LC, this value was also significantly lower in patients than in healthy subjects (Table 2). Interestingly, the mean hepatic systemic clearance of 18F-FDGal (
) and corrected for the individual LC, this value was also significantly lower in patients than in healthy subjects (Table 2). Interestingly, the mean hepatic systemic clearance of 18F-FDGal (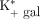 ), as derived from the PET data and arterial blood concentrations of 18F-FDGal, was not significantly different from the mean value in healthy subjects (3). Accordingly, if not corrected by the correct LC (Eq. 4), the calculation of
), as derived from the PET data and arterial blood concentrations of 18F-FDGal, was not significantly different from the mean value in healthy subjects (3). Accordingly, if not corrected by the correct LC (Eq. 4), the calculation of  would have provided erroneously high values in the patients with cirrhosis when compared with the healthy subjects.
would have provided erroneously high values in the patients with cirrhosis when compared with the healthy subjects.
The LC was significantly lower than unity in both patients with cirrhosis and healthy subjects as a result of the high substrate specificity of galactokinase (3). Interestingly, the different affinities of galactokinase for 18F-FDGal and galactose caused the hepatic systemic clearance of 18F-FDGal to be enzyme-determined in both patients with cirrhosis and healthy subjects (3,7) whereas the hepatic systemic clearance of galactose was flow-determined in both groups of subjects. When galactose is used for measurements of hepatic metabolic function, a relatively high blood concentration of galactose is accordingly required to achieve a near-saturated hepatic removal rate of galactose to avoid the influence of possible changes in hepatic blood flow on the estimate. In contrast, the hepatic systemic clearance of 18F-FDGal is enzyme-determined in both patients with cirrhosis and healthy subjects (3,7). The measured hepatic systemic clearance of 18F-FDGal obtained from the PET study without infusion of galactose (i.e.,  ) accordingly approximates the hepatic intrinsic clearance of 18F-FDGal (
) accordingly approximates the hepatic intrinsic clearance of 18F-FDGal (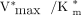 ) and not flow. The Michaelis constant for 18F-FDGal (
) and not flow. The Michaelis constant for 18F-FDGal ( ) cannot be determined independently, only as the ratio
) cannot be determined independently, only as the ratio  (3,7). Since Km for galactose was similar in healthy subjects and patients with cirrhosis (Table 1), it is unlikely that
(3,7). Since Km for galactose was similar in healthy subjects and patients with cirrhosis (Table 1), it is unlikely that  should differ between the 2 groups of subjects.
should differ between the 2 groups of subjects.  accordingly reflects
accordingly reflects  and thus provides a measurement of the hepatic galactokinase capacity for 18F-FDGal without the need for correction by the LC. In accordance,
and thus provides a measurement of the hepatic galactokinase capacity for 18F-FDGal without the need for correction by the LC. In accordance,  was significantly lower in patients with cirrhosis than in healthy subjects (3,7). These kinetic features also mean that if the same patient is followed over time, any observed changes in
was significantly lower in patients with cirrhosis than in healthy subjects (3,7). These kinetic features also mean that if the same patient is followed over time, any observed changes in  reflect changes in
reflect changes in  and not changes in
and not changes in  or hepatic blood flow.
or hepatic blood flow.
The present results demonstrated the importance of applying the correct LC in PET studies in which a tracee is present in significant concentrations compared with the tracer. This finding does not affect the use of 18F-FDGal as a PET tracer of hepatic metabolic function because the kinetic properties of the tracer are so favorable that the investigation can be performed without coadministration of galactose, that is, tracer only. This abolishes the need for correcting the 18F-FDGal clearance measurements by LC since galactose is not present in blood during fasting. However, for other tracers such as the glucose analog 18F-FDG, it is important to know the correct LC because of the presence of glucose in blood. The mean LC for 18F-FDGal was 0.14 in healthy anesthetized pigs (17), which is not significantly different from the mean LC of 0.13 in healthy human subjects (3). For other tracers, species differences must be considered a possibility, especially in disease, and determination of the LC in human subjects should therefore be preferred. Furthermore, for an organ such as the brain, which comprises functionally and structurally different structures, potential regional differences in the LC of the PET tracer must also be considered (2).
CONCLUSION
The LC for a PET tracer can be significantly affected by disease, as shown in the present study in which the LC for 18F-FDGal in patients with liver cirrhosis was significantly higher than the mean LC previously determined in healthy subjects. The results underline the importance of applying the correct LC when performing PET studies; an LC for a PET tracer determined in healthy subjects cannot be uncritically applied to patients, and even for patients, a universal mean value may not be possible to obtain. However, the kinetic properties of 18F-FDGal make it possible to perform PET studies of hepatic metabolic function in terms of 18F-FDGal Vmax in the liver parenchyma (∼ ) without correction by LC.
) without correction by LC.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. This study was supported in part by the Danish Council for Independent Research (Medical Sciences, 09-067618 and 09-073658), NIH (R01-DK074419), the A.P. Møller Foundation for the Advancement of Medical Science, the Novo Nordisk Foundation, Aase and Ejnar Danielsen’s Foundation, and Christian and Ottilia Brorson’s Foundation. No other potential conflict of interest relevant to this article was reported.
Footnotes
↵* Contributed equally to this work.
Published online Mar. 3, 2014.
- © 2014 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication May 7, 2013.
- Accepted for publication October 17, 2013.







