Abstract
Vascular adhesion protein-1 (VAP-1) is an endothelial glycoprotein mediating leukocyte trafficking from blood to sites of inflammation. BTT-1023 is a fully human monoclonal anti-VAP-1 antibody developed to treat inflammatory diseases. In this study, we preclinically evaluated radioiodinated BTT-1023 for inflammation imaging. Methods: Rabbits were intravenously injected with radioiodinated BTT-1023. Distribution and pharmacokinetics were assessed by PET/CT up to 72 h after injection. Human radiation dose estimates for 124I-BTT-1023 were extrapolated. Additionally, rabbits with chemically induced synovitis were imaged with 123I-BTT-1023 SPECT/CT. Results: Radioiodinated BTT-1023 cleared rapidly from blood circulation and distributed to liver and thyroid. Inflamed joints were delineated by SPECT/CT. The estimated human effective dose due to 124I-BTT-1023 was 0.55 mSv/MBq, if blockage of thyroid uptake is assumed. Conclusion: The radioiodinated BTT-1023 was able to detect mild inflammation in vivo. Clinical 124I-BTT-1023 PET studies with injected radioactivity of 0.5–0.7 MBq/kg may be justified.
Vascular adhesion protein-1 (VAP-1) is an endothelial glycoprotein that plays a key role in cellular trafficking by recruiting leukocytes from blood into lymphoid organs and inflamed tissues (1–3). VAP-1 is practically absent from the endothelial surface of normal tissues, but on inflammation, it is translocated from intracellular storage granules to the endothelial cell surface (2). For instance, dermal blood vessels in various inflammatory skin diseases (e.g., psoriasis) and synovial blood vessels in inflamed joints (e.g., rheumatoid arthritis) express VAP-1 on their surface (4–7). Besides being an adhesion molecule, VAP-1 also is a semicarbazide-sensitive amine oxidase. The end products of the reaction are highly potent inflammatory mediators. Blocking of VAP-1 reduces leukocyte adhesion, suggesting that VAP-1 is a potential target for antiinflammatory therapy (8).
PET with monoclonal antibodies, immuno-PET, can be applied to quantify expression of accessible antigens in the target tissue. Potentially, this specific application of PET could help in stratifying patients for antibody-based therapies. Another important application of immuno-PET could be its use in the development of antibodies as drugs. PET, using therapeutic antibodies labeled with, for example, 124I or 89Zr, may provide useful quantitative information about pharmacokinetics, accumulation in targeted and nontargeted tissues, and saturation of the target antigen in patients. Immuno-PET may assist in accurate dosing of novel biologicals, possibly speeding the development of therapeutic antibodies, their fragments, or fusion proteins (9). Imaging agents targeting VAP-1 might be valuable not only for the diagnosis and planning of treatment in patients but also for in vivo imaging of leukocyte trafficking at sites of inflammation.
BTT-1023 is a recombinant fully human monoclonal antibody directed to human VAP-1. It belongs to the IgG4 class and carries intentional mutations to remove unfavorable Fc receptor binding properties and to stabilize the dimer (10). BTT-1023 binds to human VAP-1, thereby blocking its function as an adhesion molecule. It also recognizes VAP-1 of other primates and rabbits but does not cross-react with rat or mouse. BTT-1023 is potentially useful for the treatment and in vivo imaging of inflammatory conditions such as rheumatoid arthritis.
The purpose of this study was to obtain preclinical information on the whole-body distribution, pharmacokinetics, and inflammation detection of intravenously administered radioiodinated BTT-1023 in rabbits. For potential clinical use, human radiation doses of 124I-BTT-1023 were estimated from the rabbit data.
MATERIALS AND METHODS
Additional details on the methods are provided as supplemental data (available at http://jnm.snmjournals.org).
Radioiodination and VAP-1 Binding of BTT-1023
BTT-1023 was radioiodinated using the chloramine-T method followed by purification with size-exclusion chromatography. Radiochemical purity was analyzed using thin-layer chromatography.
Binding of radioiodinated BTT-1023 to VAP-1 was evaluated using human VAP-1–transfected cells versus mock-transfected cells and radioimmunoassay or flow cytometry.
Animals
Selection of rabbits as the experimental animal was based on BTT-1023 cross-reactivity. All studies were conducted with approval from the Lab-Animal Care and Use Committee of the State Provincial Office of Southern Finland and in compliance with Finnish laws relating to the conduct of animal experimentation. In total, 14 rabbits (2.4–4.0 kg) were used in the studies. For imaging, the rabbits were sedated and anesthetized. To induce synovitis, 4 rabbits were intraarticularly injected with phytohemagglutinin (11).
PET Studies
124I-BTT-1023 (52 ± 2.5 MBq) was injected intravenously, and whole-body PET/CT scanning was performed over the first 2 h and at 24, 48, and 72 h after injection (Discovery VCT; GE Healthcare). The radioactivity concentration in various organs was analyzed, and the results were expressed as standardized uptake values. During PET, serial blood samples were collected. The amount of intact 124I-BTT-1023 as a function of time after injection was used for the calculation of pharmacokinetic parameters. After the last PET/CT scan, that is, 72 h after injection, various organs were excised, weighed, and measured for radioactivity using a γ-counter.
Distribution of radioactivity in several organs was also studied by autoradiography of cryosections. After autoradiography, adjacent sections were stained with anti-VAP-1 antibody and with hematoxylin–eosin.
Human radiation dose estimates were extrapolated from the rabbit data using OLINDA/EXM software.
SPECT/CT
123I-BTT-1023 (22 ± 9.3 MBq) was injected intravenously, and SPECT/CT scanning was performed at 2 h after injection (Symbia TruePoint; Siemens Healthcare). The radioactivity concentration in the inflamed knee, the contralateral knee, and the contralateral muscle was analyzed.
Statistics
All results are expressed as mean ± SD. Statistical analyses were performed with the Student t test and linear regression analysis. A P value of less than 0.05 was considered statistically significant.
RESULTS
Inflammation Model
Lymphocyte infiltrations are characteristic of inflamed human synovium, such as in rheumatoid arthritis (Fig. 1A). A rabbit model of acute synovial inflammation was generated using chemical induction. After phytohemagglutinin challenge, all rabbits had swollen and warm joints as symptoms of inflammation. Closer histologic examination revealed only mild inflammation, with occasional granulocytes in the inflamed knee in comparison to the control knee (Figs. 1B and 1C). Unlike the human inflamed synovium, which has abundant expression of VAP-1 on the vasculature (Fig. 1D), VAP-1 induction was minor in this acute inflammatory state (Fig. 1E). Unexpectedly, there were also VAP-1–positive vessels in the healthy contralateral side (Fig. 1F). This finding could have been due to a systemic response to phytohemagglutinin injected in the knee cavity or VAP-1 expressed constitutively in the joint vasculature of the rabbit.
Induction of VAP-1 in rabbit and human synovial inflammation. (A–C) Hematoxylin–eosin stainings of inflamed human synovium (A), inflamed rabbit synovium (B), and synovium of control knee of rabbit (C). (D–F) Immunohistochemical stainings of VAP-1 in inflamed human synovium (D), inflamed rabbit synovium (E), and synovium of control knee of rabbit (F). Insets show stainings with control antibody. Some positive (red) vessels are indicated by arrows. Magnification is ×100.
VAP-1 Binding of Radioiodinated BTT-1023
According to radioimmunoassay, binding of 124I-BTT-1023 to human VAP-1–transfected cells was approximately 600-fold higher than binding to mock-transfected cells, and binding was eliminated in competition assays using an excess of unlabeled antibody (Supplemental Fig. 1A). Also, in flow cytometric analyses, 100% of human VAP-1–transfected cells had positive 123I-BTT-1023 staining, whereas mock-transfected cells remained negative (Supplemental Fig. 1B).
PET Studies
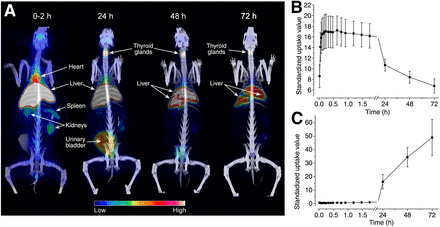
Representative PET/CT images and time–activity curves of liver and thyroid glands are presented in Figure 2 and Supplemental Table 1. Radioactivity accumulated in the liver rapidly after injection and declined slowly during the 4-d experiment. The thyroids showed high uptake at day 4, which was probably due to the deiodination of the antibody. Radioactivity cleared rapidly from the heart. Only a small amount of radioactivity was present in the kidneys, which function as an elimination route for free iodine.
Whole-body distribution of 124I-BTT-1023 in healthy rabbit. (A) PET/CT images showing distribution of radioactivity up to 72 h after injection. (B and C) Time–activity curves of liver (B) and thyroid glands (C).
Ex vivo measurements confirmed the in vivo imaging results at 72 h after injection (Supplemental Table 2; Supplemental Fig. 2). The highest 124I-radioactivity concentrations were in the thyroid and the liver. All other organs had a radioactivity concentration below 0.1 percentage injected radioactivity per gram of tissue. The brain had the lowest uptake. Linear regression analysis showed a strong correlation between the radioactivity of tissue samples and PET values (R = 0.94; P < 0.0001) (Supplemental Fig. 2B).
In small-intestine cryosections, the radioactivity concentrated in the smooth muscle rather than in the lining or mucosal part of the intestine. These observations are in line with anti-VAP-1 immunohistochemistry, which demonstrates expression of VAP-1 both in the smooth muscle cell layer and in the subpopulation of mucosal vessels (Figs. 3A–3C). According to the autoradiography of small-intestine cryosections, uptake of 124I-BTT-1023 was significantly higher in VAP-1–positive areas than in VAP-1–negative areas, at 4.8 ± 2.1 and 0.93 ± 0.61 photostimulated luminescence per square millimeter, respectively (P < 0.001; Fig. 3D).
Binding of 124I-BTT-1023 to VAP-1–positive vessels in small intestine. (A–C) Hematoxylin–eosin staining (A), autoradiograph (B), and anti-VAP-1 immunohistochemical staining (C) of small-intestine cryosections. (D) Distribution of radioactivity at 72 h after injection in VAP-1–negative and VAP-1–positive areas of small intestine (Student t test).
The human radiation dosimetry estimates extrapolated from the rabbit data are presented in Table 1. The mean effective dose due to 124I-BTT-1023 was 0.55 mSv/MBq, if one assumes blockage of thyroid uptake. Importantly, only minimal radioactivity was found in bone marrow.
Human Radiation Dosimetry Extrapolated from Rabbit 124I-BTT-1023 Data
Further details on the results are provided as supplemental data.
SPECT/CT
Inflamed rabbit knee visualized with SPECT/CT had inflammation-to-control joint and inflammation-to-muscle ratios of 1.2 ± 0.1 and 2.2 ± 1.0, respectively (Fig. 4). In vivo SPECT results were confirmed by ex vivo measurements demonstrating inflammation-to-control joint and inflammation-to-muscle ratios of 1.7 ± 0.5 and 3.3 ± 2.4, respectively.
123I-BTT-1023 detection of synovial inflammation in rabbit knee. Transaxial SPECT (A), SPECT/CT (B), and CT (C) images show localization of radioactivity in inflamed knee (red arrow) and control knee (white arrow) at 2 h after injection.
DISCUSSION
We demonstrated that the fully human antibody BTT-1023 can be labeled with both 124I and 123I without eliminating its affinity for human VAP-1. The whole-body distribution and pharmacokinetics of intravenous 124I-BTT-1023 were elucidated by PET/CT of healthy rabbits. In addition, we showed that 123I-BTT-1023 SPECT/CT can detect inflammation in a rabbit model of arthritis.
The high liver uptake is probably mediated in large part by VAP-1, since the antigen is found on the sinusoidal endothelia (12). However, a minor part of the signal is likely caused by the hepatic elimination of the antibody. Previously, Jaakkola et al. studied the in vivo behavior of 123I-labeled chimeric mouse–human anti-VAP-1 antibody in dogs and pigs using SPECT (11). Their results suggest that uptake in liver is VAP-1–specific. Imaging of inflammation in the abdominal area is limited, since the liver shows high uptake. Thyroid radioactivity is most plausibly due to the deiodination of 124I from the antibody. Free iodine is rapidly taken up by thyroid glands, and the excess is excreted via the kidneys to the urine.
In vivo imaging of synovial inflammation was demonstrated in an animal model of mild arthritis. Although the difference between affected and control joints was modest, similar ratios have been found with 18F-FDG in the same animal model (Autio et al., unpublished data, 2012). The current detection of even very mild inflammation indicates the imaging potential of this tracer. The expression of VAP-1 and its regulation are not identical in humans and rabbits. Intravenously injected antibody detects VAP-1 also in the control knee of rabbits, and rabbit heart endothelial cell cultures express VAP-1 on the surface, whereas in humans the surface expression of endothelial VAP-1 is not detectable in noninflamed skin and human endothelial cell cultures do not express VAP-1 on the surface (6,13).
The estimated mean effective dose for a 70-kg man resulting from 124I-BTT-1023 (3.66 mSv/MBq) is rather high. However, with effective blocking of the thyroid gland by an adequate regimen, the estimated human radiation dose would decrease to a more acceptable level (0.55 mSv/MBq). Clinical PET imaging and dose estimations of several 124I-labeled substances have been reported by other researchers (14–18).
To obtain proper PET images, the injected radioactivity of 124I would ideally need to be higher than that of other PET radionuclides since only 25% of the 124I decay is by positron emission, with the rest being γ- and x-rays. On the other hand, the longer physical half-life of 124I facilitates longer acquisition of PET data, improving the image quality. Detection of 124I-BTT-1023—for example, in an inflamed human joint—should be possible with injected radioactivity of 0.5–0.7 MBq/kg since there is less background radioactivity, scatter, and random radioactivity in the vicinity. Moreover, the joint is reasonably easy to stabilize to minimize signal noise.
In general, imaging agents targeting VAP-1 could be valuable not only for the diagnosis and planning of treatment for patients with inflammatory conditions, such as rheumatoid arthritis, but also for the drug discovery and development processes. In addition to therapy monitoring, VAP-1 imaging would provide a scientific tool for in vivo imaging of leukocyte trafficking at the sites of inflammation. In vivo imaging of inflammation using VAP-1 as a target molecule and radiolabeled BTT-1023 as a tracer, such as in patients with rheumatoid arthritis, could help in stratifying patients for BTT-1023 antiinflammatory treatment.
CONCLUSION
BTT-1023 retained its biologic activity to bind VAP-1 after radioiodination in both in vitro and in vivo settings, and PET/CT imaging elucidated the whole-body distribution and pharmacokinetics over 4 d in rabbits. Most importantly, radiolabeled BTT-1023 was able to visualize mildly inflamed rabbit joints in SPECT/CT. There is a need for further studies with inflammation models to validate its future use in different clinical settings.
DISCLOSURE
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734. The study was conducted within the Finnish Center of Excellence in Molecular Imaging in Cardiovascular and Metabolic Research supported by the Academy of Finland, University of Turku, Turku University Hospital, and Åbo Akademi University. Petri J. Vainio and Jani Vainio are employees of Biotie Therapies Corp. Antti Mali is an employee of MAP Medical Technologies. No other potential conflict of interest relevant to this article was reported.
Acknowledgments
English language proofreading of this article was kindly performed by Robert M. Badeau, PhD. Tuomo Nikula, Heidi Liljenbäck, and Erica Nyman are acknowledged for technical assistance.
Footnotes
Published online Jul. 11, 2013.
- © 2013 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
REFERENCES
- Received for publication January 23, 2013.
- Accepted for publication March 20, 2013.