Abstract
Inflammation and angiogenesis are hypothesized to be important factors contributing to plaque vulnerability, whereas calcification is suggested to confer stability. To investigate this in vivo, we combined CT angiography and PET and compared the findings with immunohistochemistry for patients undergoing carotid endarterectomy. Methods: Twenty-one consecutive patients (18 men, 3 women; mean age ± SD, 68.3 ± 7.3) undergoing carotid endarterectomy were recruited for combined carotid 18F-FDG PET/CT angiography. Plaque 18F-FDG uptake was quantified with maximum standardized uptake value, and CT angiography quantified percentage plaque composition (calcium and lipid). Surgical specimens underwent ex vivo CT aiding image registration, followed by immunohistochemical staining for CD68 (macrophage density) and vascular endothelial growth factor (angiogenesis). Relationships between imaging and immunohistochemistry were assessed with Spearman rank correlation and multivariable regression. Results: The mean (±SD) surgically excised carotid plaque 18F-FDG metabolism was 2.4 (±0.5) versus 2.2 (±0.3) contralaterally (P = 0.027). There were positive correlations between plaque 18F-FDG metabolism and immunohistochemistry with CD68 (ρ = 0.55; P = 0.011) and vascular endothelial growth factor (ρ = 0.47; P = 0.031). There was an inverse relationship between plaque 18F-FDG metabolism and plaque percentage calcium composition on CT (ρ = −0.51; P = 0.018) and between calcium composition and immunohistochemistry with CD68 (ρ = −0.57; P = 0.007). Regression showed that maximum standardized uptake value and calcium composition were independently significant predictors of angiogenesis, and calcium composition was a predictor of macrophage density. Conclusion: We provide in vivo evidence that increased plaque metabolism is associated with increased biomarkers of angiogenesis and inflammation, whereas plaque calcification is inversely related to PET and histologic biomarkers of inflammation.
The identification of atheroma at risk of causing cardiovascular events has been investigated for many years (1). The role of imaging in this respect has been 2-fold: first, to anatomically characterize atheroma with features of vulnerability, and second, to investigate key components of plaque pathogenesis such as inflammation and angiogenesis (2). PET (3,4) and dynamic MRI (5) have been used to image the latter, whereas high-resolution MRI (6) and CT (7,8) have been used to characterize plaque morphology. CT is particularly of interest because this is an ideal imaging modality for coronary arteries, and there is evidence that calcium composition predicts the likelihood of acute coronary syndromes (9).
To synergistically image plaque vulnerability, it may be desirable to use a multimodality approach so that anatomy and pathophysiology may be assessed together. The value of such an approach has been shown in oncology (10) and cardiology (11). Indeed, this combined approach has now been applied to carotid plaque imaging (12,13). However, the findings have been conflicting with respect to the relationship between uptake on PET and anatomic imaging: lipid-rich necrotic core plaques demonstrated higher 18F-FDG uptake than calcified or collagen plaques in one study (12), but no strong correlations between 18F-FDG uptake and the CT- or MRI-assessed composition of the plaques existed in another (13). Moreover, gold standard histologic comparison was unavailable for either of these studies. Histologic comparison may provide important insight into atheroma pathogenesis.
Therefore, in this study, we combined carotid plaque 18F-FDG PET/CT with carotid CT angiography in patients undergoing carotid endarterectomy and performed bioassays of macrophage density (CD68; Dako) and angiogenesis (vascular endothelial growth factor [VEGF]; Dako) in the resected specimens.
MATERIALS AND METHODS
After approval from the institutional ethics committee, 21 consecutive patients (18 men, 3 women; mean age ± SD, 68.3 ± 7.3 y) undergoing carotid endarterectomy were recruited for combined carotid 18F-FDG PET/CT angiography. All patients had 70% or greater carotid stenosis as determined by duplex ultrasound. All patients gave informed consent. The patients’ clinical characteristics are shown in Supplemental Table 1 (available online only at http://jnm.snmjournals.org).
Image Acquisition
18F-FDG PET/CT
After a 6-h fast, patients received an intravenous injection of 190 MBq of 18F-FDG. The mean 18F-FDG uptake time was 92.4 ± 4.6 min (14). Using a Discovery LS PET/CT scanner (GE Healthcare), we imaged a single bed position (148.75 mm) centered on the carotid bifurcation, with the patients supine with their arms positioned beside their torso. CT for attenuation correction was performed: 140 kVp and 80 mAs; tube rotation time, 0.8 s; detectors, 64 × 0.625 mm; pitch, 1.5; and collimation, 5 mm. Maintaining patient position, we obtained a PET scan covering an area identical to that covered by CT. All images were acquired in 2-dimensional mode, consisting of an emission scan of 10 min. PET images were reconstructed with CT for attenuation correction using CT maps. Transaxial emission images of 3.9 × 3.9 × 4.25 mm (in-plane matrix size, 128 × 128) were reconstructed using ordered-subsets expectation maximization with 2 iterations and 28 subsets. The z-axis coverage was 148.75 mm, resulting in 35 slices.
CT Carotid Angiography
Immediately after PET, CT carotid angiography was performed from the aortic arch to the base of skull, using 50 mL of intravenous contrast medium (Omnipaque 350 [GE Healthcare]; 5 mL/s), followed by 50 mL of normal saline, with the patient maintained in the same position. Scanning parameters were 120 kVp; 150 mAs; tube rotation time, 0.5 s; detectors, 64 × 0.625 mm; pitch, 0.984; and collimation, 2.5 mm. The field of view was 250 × 250 mm, and matrix size was 512 × 512. CT images were reconstructed with a 0.625-mm slice thickness and no interval.
Image Analysis
All images were analyzed using the carotid bifurcation as an anatomic landmark to allow direct comparison of histology with 18F-FDG PET/CT and CT angiography.
18F-FDG PET/CT
Images were reported in consensus by a dual-accredited nuclear medicine physician–radiologist with a cardiovascular interest (>5 y experience, with >4 y experience at quantifying carotid plaque 18F-FDG uptake) and a radiologist with a cardiovascular and PET special interest (>4 y experience at quantifying carotid plaque 18F-FDG uptake). Images were loaded on an Advantage Workstation (GE Healthcare). Regions of interest were drawn on the carotid artery wall at the bifurcation, and the maximum standardized uptake value (SUVmax) was recorded. The SUVmax was normalized to the blood-pool SUVmax value measured from the internal jugular vein, giving the tissue-to-background ratio.
CT Angiography
One investigator, a radiologic technologist with more than 10 y of experience in CT angiography, unaware of the PET/CT findings, evaluated the CT angiograms of the surgical side. Images were loaded onto an Advantage Workstation. Colorimetric analysis of the carotid bifurcation was used to quantify different plaque components. Different Hounsfield unit (HU) ranges were considered to represent different plaque components: calcification, greater than 600 HUs, and lipids, 20–60 HUs (7,8). Figures 1B and 2B show examples of the colorimetric analysis drawn on the CT images. The software calculated the percentage plaque composition with lipids and calcification. To determine the variability of the plaque composition measurement, the images were reanalyzed by the same observer, 3 mo apart in a masked manner. The intraclass correlation coefficients were 0.996 (95% confidence interval, 0.989–0.999) for percentage calcification and 0.965 (95% confidence interval, 0.901–0.988) for percentage lipids.
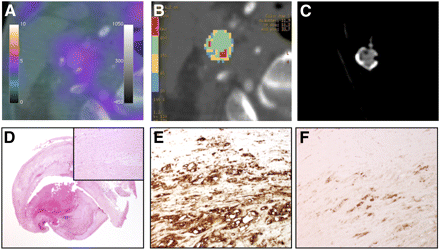
(A) Contrast-enhanced PET/CT scan showing carotid bifurcation with increased 18F-FDG uptake (scale bar on left, SUVmax; scale bar on right, CT window level and width). (B) Colorimetric analysis of plaque component areas on CT scan. Ranges of HU values represent different plaque components: azure, lipid core (20–60 HU), and red, calcification (600–2,000 HU). (C) Axial CT scan of ex vivo carotid artery specimen. (D) Hematoxylin and eosin low-power magnification (×12.5) and detail magnification (×200) of same carotid bifurcation. (E) CD68-stained (×200) section of carotid bifurcation. (F) VEGF-stained (×200) section of carotid bifurcation.
(A) Contrast-enhanced PET/CT scan showing carotid bifurcation with low 18F-FDG uptake (scale bar on left, SUVmax; scale bar on right, CT window level and width). (B) Colorimetric analysis of plaque component areas on CT. Ranges of HU values represent different plaque components: azure, lipid core (20–60 HU), and red, calcification (600–2,000 HU). (C) Axial CT scan of ex vivo carotid artery specimen. (D) Hematoxylin and eosin low-power magnification (×12.5) and detail magnification (×200) of same carotid bifurcation. (E) CD68-stained (×200) section of carotid bifurcation. (F) VEGF-stained (×200) section of carotid bifurcation.
Image Registration
The PET and CT angiography images were automatically coregistered by the Advantage Workstation (Figs. 1A and 2B).
Histologic Preparation and Analysis
All patients were operated on within 2 wk of imaging. The endarterectomy specimens were fixed in 10% formalin. To maximize in vivo registration with histologic sections, the resected endarterectomy specimens underwent ex vivo CT examination before sectioning (Figs. 1C and 2C). Using the carotid bifurcation as the anatomic landmark, the radiologist and histopathologist in consensus carefully coregistered the endarterectomy specimens to the carotid plaque images (in vivo CT, CT angiography, and ex vivo CT images). In all patients, histologic analyses were performed at the carotid bifurcation for direct comparison of PET and CT angiography.
Histologic sections (4 μm thick) from each tissue block in each patient were stained with hematoxylin and eosin and monoclonal antibodies CD68 (dilution, 1/50) for macrophages (3) and VEGF (dilution, 1/100) for angiogenesis (15). Immunohistochemical staining of tissue sections was performed on the fully automated Bond-maX system (Leica Microsystems) and was used in conjunction with the Bond Polymer Refine system. All sections were stained at the same time.
Automated image quantification was performed using digital microscopy to analyze the immunohistochemical staining. All the slides were scanned using Mirax Scan (Carl Zeiss), and the images provided by the software were exhibited on a liquid crystal display monitor under contrast, focus, saturation, and white balance standardization. To evaluate the staining intensity, an image analysis system (HistoQuant; 3DHistech) was used. The software identified the immunohistochemical staining to be quantified by minimizing background-staining artifacts using image filters. Because the software recognized the positive nuclei staining of all different intensities, the quantification was processed in each tissue microarray section (spot) automatically by the software. Each spot's numeric data of staining intensity average was exported to a Microsoft Excel file.
CD68-stained macrophages were expressed as a percentage of plaque area. In cases for which the plaque occupied more than 180° of the vessel wall, the value for percentage plaque CD68 staining in the most inflamed half of the vessel wall was recorded (4). VEGF staining of endothelial cells was expressed as a score of number of stained cells and intensity.
Statistical Analysis
A paired t test was used to compare the PET findings between the plaque surgically operated on and the contralateral carotid plaque. The intraclass correlation coefficient was used to assess intraobserver variability of CT plaque composition measurements. Spearman correlation coefficients (ρ) were calculated for the association between imaging parameters and immunohistochemistry. To check whether any of the imaging parameters were independently statistically significant, they were introduced in a multiple regression model, and backward stepwise regression analysis was performed. All statistical tests were performed using software from GraphPad (Prism and InStat).
RESULTS
The mean (±SD) surgical carotid plaque 18F-FDG metabolism (SUVmax) was 2.4 ± 0.5 versus 2.2 ± 0.3 contralaterally (P = 0.027). There was no significant difference in mean surgical carotid plaque tissue-to-background ratio versus contralaterally (P = 0.264).
Comparison Between 18F-FDG PET and CT Angiographic Findings
The correlation between plaque 18F-FDG metabolism and percentage plaque calcification on CT angiography was −0.51 (P = 0.018) (Fig. 3). The correlation between plaque 18F-FDG metabolism and percentage plaque lipid on CT angiography was 0.13 (P = 0.569).
Scatterplot to show Spearman rank correlation of SUVmax with percentage plaque composition by calcium.
Comparison of 18F-FDG PET Findings with Histology
The correlation between plaque 18F-FDG metabolism and plaque CD68 expression on immunohistochemistry was 0.55 (P = 0.011) (Fig. 4). The correlation between plaque 18F-FDG metabolism and plaque VEGF expression on immunohistochemistry was 0.47 (P = 0.03) for VEGF.
Scatterplot to show Spearman rank correlation of SUVmax with plaque staining intensity with CD68.
Comparison of CT Angiographic Findings with Histology
The correlation between plaque calcium composition and plaque CD68 expression on immunohistochemistry was −0.57 (P = 0.007). The correlation between plaque calcium composition and plaque VEGF expression on immunohistochemistry was −0.03 (P = 0.902). The correlation between plaque lipid composition and plaque CD68 expression on immunohistochemistry was −0.04 (P = 0.853). The correlation between plaque lipid composition and plaque VEGF expression on immunohistochemistry was −0.06 (P = 0.790).
Regression Analysis
When all imaging parameters were introduced into a multivariable regression model and backward stepwise regression was performed, percentage calcification remained statistically significant (P < 0.05) for CD68 expression, and SUVmax and percentage calcification remained statistically significant (P < 0.05) for VEGF expression (Table 1).
Results of Multivariable Linear Regression Analyses, with CD68 and VEGF as Dependent Variables of Interest and Percentage Calcium and SUVmax as Statistically Significant Predictors
DISCUSSION
In this study, morphologic composition on CT with respect to increasing arterial calcification reflected underlying hypometabolism as measured by 18F-FDG PET. Furthermore, plaque calcification was inversely related to a bioassay of macrophages, whereas plaque with high 18F-FDG metabolism was associated with a raised bioassay of macrophages. These findings support current evidence that increased 18F-FDG plaque metabolism is associated with instability (2,3), whereas increased plaque calcification confers stability (16).
Our PET/CT angiography findings showed an inverse relationship between 18F-FDG arterial uptake on PET and arterial calcification. The inverse relationship would help explain why vascular 18F-FDG uptake and calcification rarely overlap (17,18). This negative correlation supports the view that calcification represents a late stage of atherosclerosis (19,20)—important with respect not only to carotid artery disease but also to coronary artery disease. Coronary arteries are difficult to image using PET because of their size and cardiac motion. In contrast, CT is an ideal coronary artery imaging technique. Potentially, differences in plaque composition on CT angiography may be useful for the noninvasive identification of atherosclerotic plaques associated with higher risk (9,21), for which one would expect that plaques with spotty calcification (16) would overlap with the plaques we identified with little percentage calcification by composition and inflammation by metabolism. The histologic findings in our study in relation to plaque composition would seem to support this hypothesis. Whether plaque characterization on PET/CT angiography could be translated into medical practice remains to be seen. Currently, risk stratification is based on clinical data. PET/CT angiography may recognize plaques at higher risk, according to their composition and metabolism. However, we await prognostic data from large outcome studies (22), and improvements in the imaging technology, as alluded to in the study limitations.
The finding that there is a relationship between morphologic plaque composition and 18F-FDG PET is confirmed by others using MRI, with lipid-rich plaques having more 18F-FDG uptake than either collagen-rich or calcified plaques (12). This finding, however, has not been universal, with another group finding no correlation between standardized uptake value and calcification (13). Nonetheless, our study findings are supported further by our immunohistochemical analysis, with which we were also able to show inverse relationships between plaque calcium content and the bioassay of plaque inflammation (CD68). CD68 is a marker for macrophage activity and has been shown by histologic studies to be raised in vulnerable carotid plaque (3,23). The fact that we found statistically significantly lower levels of CD68 in calcified plaque would support the hypothesis that such plaques may be more stable.
The bioassay findings in relation to 18F-FDG PET are interesting, especially because there has been a paucity of histologically validated carotid PET plaque studies (2,3,23). We found that VEGF expression in plaque is related to 18F-FDG uptake. VEGF, an inducer of angiogenesis, may enhance plaque formation and destabilization in vulnerable atherosclerotic plaque (24). Indeed, angiogenesis is believed to be a central component in the pathogenesis of unstable atheroma (2). It would seem that a variety of cellular components and molecular mechanisms within the endothelial lesion, including macrophage infiltration and angiogenesis, may contribute to 18F-FDG uptake in inflamed atherosclerotic plaque (25).
When performing carotid imaging studies, there are several methodologic considerations of which to be aware. There is a difference regarding the precise methodologic details of arterial imaging with 18F-FDG PET (26). Some groups favor SUVmax measurements (27), others standardized uptake value normalized to blood (target to background ratio) (3) or whole-vessel analysis (14). SUVmax measurements have shown to be the most reproducible 18F-FDG measurements in carotid arteries (28). The lack of difference in carotid tissue-to-background ratio between the operated side and nonoperated side may be due to the extra variability introduced by normalizing the uptake. Alternatively, given that the degree of uptake in the nonoperated side was greater than has been described in healthy populations (19,29), the lack of difference may reflect the possibility that the pathophysiologic processes behind plaque vulnerability also exert effects throughout the vascular tree, as has been shown in the coronary circulation (30).
In our study, we obtained SUVmax measurements at the carotid bifurcation to enable precise imaging–histologic correlation. Attention to detail was made to maximize image registration of morphology to histology. Nevertheless, the tissue thickness encompassed by imaging (mm) is different from the tissue thickness covered by immunohistochemistry (μm), and this difference is an inherent limitation of all such published studies (2–4,27). All published histologically validated carotid plaque studies have limited-size populations, with heterogeneity in terms of symptoms, time to surgery, and medications (2–4,31), reflecting the logistic difficulties of these types of studies.
CONCLUSION
We provide in vivo evidence that increased plaque metabolism is associated with increased biomarkers of angiogenesis and inflammation, whereas plaque calcification is inversely related to PET and histologic biomarkers of inflammation. Whether these findings can be translated into the clinic with respect to risk stratification or treatment monitoring should be addressed.
DISCLOSURE STATEMENT
The costs of publication of this article were defrayed in part by the payment of page charges. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr. Gareth Ambler, Biostatistician, Joint UCL/UCLH Biomedical Research Unit, and Dr. Xanthi Pedeli, University of Athens, for their statistical advice. We acknowledge the input of Professor Brian F. Hutton, UCL, regarding image coregistration and 18F-FDG uptake measurements. This study was funded in part by contributions from the Sussex Stroke and Circulation Fund and the Royal College of Radiologists. UCL/UCLH receives a proportion of funding from the Department of Health's NIHR Biomedical Research Centre's funding scheme. No other potential conflict of interest relevant to this article was reported.
Footnotes
Published online Oct. 11, 2011.
- © 2011 by Society of Nuclear Medicine
REFERENCES
- Received for publication May 25, 2011.
- Accepted for publication August 17, 2011.