Abstract
The radioligand [carbonyl-11C]WAY-100635 (11C-WAY) is a PET tracer of the serotonin 5HT1A receptors in the human brain. It is metabolized so rapidly in the circulation that it behaves more as a chemical microsphere than as a tracer subject to continuous exchange between the circulation and brain tissue. Although reference tissue methods are useful as analyses of uptake of some radioligands with indeterminate arterial input functions, their use to analyze 11C-WAY uptake and binding is challenged by the rapid plasma metabolism, which violates the assumption that regions of interest and reference regions continue to exchange radioligand with the circulation during the entire uptake period. Here, we proposed a method of calculation (Hypotime) that specifically uses the washout rather than the accumulation of 11C-WAY to determine binding potentials (BPND), without the use of regression analysis. Methods: A total of 19 healthy volunteers (age range, 23–73 y) underwent PET to test the Hypotime application of the chemical microsphere properties of 11C-WAY to identify regions of binding and nonbinding on the exclusive basis of the rate of washout of 11C-WAY. Results: The results of the Hypotime method were compared with the simplified but multilinearized reference tissue method (MLSRTM). The distribution of receptor BPND obtained with Hypotime was consistent with previous autoradiography of postmortem brain tissue, with the highest values of BPND recorded in the medial temporal lobe and decline of receptor availability with age. The values in the basal ganglia and cerebellum were negligible. The MLSRTM, in contrast, yielded lower BPND in all regions and only weakly revealed the decline with age. Conclusion: The simple and computationally efficient Hypotime method gave reliable values of BPND without the use of regression. The MLSRTM, on the other hand, appeared to be affected by the early disappearance of the radioligand from the circulation and the associated uncertain late presence of 11C-WAY in the circulation.
The radioligand [carbonyl-11C]WAY-100635 (11C-WAY) binds to the serotonin 5HT1A receptor as an antagonist. The ligand has been used to map the neuroreceptors in healthy subjects and in patients with diseases as diverse as major depression (1), bulimia nervosa (2), amyotrophic lateral sclerosis (3), schizophrenia (4), Parkinson disease (5), and temporal lobe epilepsy (6). In general, intrasubject variability of the binding is low, but intersubjective variability is high (7,8). Although the receptor binding potentials (BPND) globally decline in depression, other diseases reveal only subtle changes, and demographic, physiologic, and psychologic measures do not consistently explain the highly variable receptor availability reported for healthy subjects. We recently reported a 3% reduction of the availability of the BPND of this receptor per decade of life in healthy volunteers (9), but the evidence for an age-related decline is still subject to uncertainty because of the wide variability of the results (7,10,11).
Studies in both humans and animals generally reveal that the BPND is insensitive to changes of the concentration of endogenous serotonin in brain tissue (1,12–14). These variable findings raise the question of whether the diversity of BPND values among people measured with 11C-WAY reflects a true heterogeneity of the density of 5HT1A receptors or whether methodologic issues concerning the correct quantification of the BPND could contribute to the variability.
Three problems complicate the correct quantification of 5HT1A receptor binding with 11C-WAY. The first of the 3 potential biases is the rapid metabolism of the tracer in the circulation (15,16). The rapid disappearance of the tracer from the circulation and the consequent brief exchange with brain tissue imply that the tracer could behave much like a chemical microsphere. Conventional tracer uptake normally occurs over a period of time as a continuous function of the magnitude of the arterial input function, whereas with microspheres, the actual arterial input function is difficult to determine because its temporal extent is limited to that of a narrow spike.
In receptor mapping, the reference tissue method is popular because it appears to eliminate the need to measure the arterial input function of a tracer, particularly in the cases of tracers that behave as chemical microspheres. However, as in the case of 11C-WAY, the rapid metabolism in turn may violate the fundamental assumption that both the reference region and the pool of free tracer in the binding regions continue to exchange significant quantities of tracer with the circulation. In the absence of a continuing source of tracer in the circulation, reference and binding regions independently clear tracer from the respective volumes of distribution, and tracer in the reference region no longer is a proper surrogate for tracer in the circulation. The time–activity functions of different regions now depend on regional properties of binding, blood flow, and blood–brain barrier permeability, rather than on a common source of tracer in the circulation.
The second potential problem is the uncertainty about a proper region of reference. The adult cerebellum is believed to be almost devoid of 5HT1A receptors (17). Although they may be present in this part of the brain in fetal and early life, the density declines with age. Recent studies of the adult brain (8,18) demonstrate specific residual binding in vermis and cortex of cerebellum that may lead to the underestimation of binding in other regions if vermis and cortex of cerebellum are included in the reference region. In 3 studies with 11C-WAY (3,5,7), as many as 10% of the subjects were excluded because of abnormally high cerebellar time–activity curves. The underlying causes were not examined, but specific binding in the cerebellum or incorrect segmentation of the cerebellum are plausible explanations.
The third potential problem is the influence of blood flow and blood–brain barrier permeability differences in regions of specific binding. If the tracer is subject to clearance from multiple compartments having pools of exchangeable tracer and of tracer bound to receptors, it is possible that both flow and permeability changes can mimic or mask changes of binding.
In the present study, to establish a method of global parametric mapping of the BPND of 11C-WAY that takes the particular kinetic properties of 11C-WAY into account, we compared 2 methods of noninvasive assay of 11C-WAY binding in the brain of healthy subjects. First, we applied a model of tracer clearance, Hypotime, which we designed to map the washout of the tracer from specifically and nonspecifically binding regions. Measures of regional tracer clearances were used to identify a reference region of negligible specific binding, The reference region subsequently was used to obtain parametric maps of BPND by means of a second method, a multilinearized simplified reference tissue method (MLSRTM), expressed as Equation 9 in a recent application of the method (19). In this method, the BPND values are estimated by conventional weighted multilinear regression of operational equations of integral form to the same regional records and as such depend on a common source of tracer in the circulation for the duration of data acquisition.
MATERIALS AND METHODS
The Research Ethics Committee of Aarhus County approved the recruitment of 19 healthy volunteers (age range, 23–73 y; 8 women, 11 men) into 2 groups, with an average age in the group of 12 elderly subjects of 62.5 ± 6.8 y (mean ± SD) and an average age in the group of 7 young subjects of 25.1 ± 2.0 y. All volunteers gave written informed consent to participate in the study. Exclusion criteria included cardiovascular disease and any history of neurologic and psychiatric disease. All subjects were physically fit, were free of prescribed medication, and did not meet the criteria for depression according to the Diagnostic and Statistical Manual of Mental Disorders (20).
Radiochemistry
The radioligand 11C-WAY was synthesized from the cyclotron-generated precursor according to the method of McCarron et al. (21). In brief, 11CO2 was collected in a stainless steel cryotrap, warmed, and then flushed with nitrogen through a small coil of 0.2-cm-outer-diameter polypropylene tubing, which had been earlier flushed with a solution of cyclohexylmagnesium chloride (500 μL of 0.5 M in tetrahydrofuran). The 11C-labeled Grignard adduct, 11C-cyclohexanecarbonyl chloride, was eluted with a solution of thionyl chloride (10 μL in 400 μL THF) into a septum-sealed vial (2 mL) containing WAY-100634 (2 mg), 50-μL THF, and triethylamine (60 μL) under a nitrogen atmosphere. The reaction proceeded in 7 min at 85°C. The crude product was diluted with 500 μL of high-performance liquid chromatography (HPLC) eluent, and purified by reversed-phase HPLC on a Ultracarb 7 ODS 30 column (250 × 10 mm; Phenomenex Ltd.), eluting with sterile ethanol:70 mM NaH2PO4 (52:48) at 6 mL/min. The fraction containing 11C-WAY-100635 (retention time, ∼10 min) was collected, evaporated to near-dryness at 90°C under vacuum, and then reformulated in sterile saline (10 mL) before it was passed through a sterile 0.22-μm filter into a sterile vial. Injected masses were 31 nmol (SEM, 5.8 nmol) and 12 nmol (SEM, 2.5 nmol) in the groups of young and elderly subjects, respectively. Specific radioactivities were 36 GBq/μmol (SEM, 7.9) in the group of young subjects and 38 GBq/μmol (SEM, 7.8) in the group of elderly subjects.
PET
Subjects were positioned in the ECAT EXACT HR47 tomograph (CTI/Siemens). After a 15-min attenuation, a single-frame scan was acquired, starting at 60,000 true counts per second after an intravenous bolus injection of 15O-H2O (500 MBq), followed by bolus injection of 11C-WAY (150–430 MBq; mean, 270 MBq; SD, 111 MBq) and the initiation of 60-min emission recordings of 22 frames in 3-dimensional mode. High-resolution T1-weighted MR images were obtained at 1.5 or 3 T (GE Sigma Systems). The summed emission recordings were automatically coregistered to the individual MRI scans using 6 parameters. Individual MR images were coregistered to a common stereotactic space (Montreal Neurologic Institute) (22) using a 12-parameter affine rigid-body transformation; registrations were automatic in the young group but manual in the elderly group because of the inadequate registration of atrophied brains. After the calculation of the final PET-Talairach transformation matrix, dynamic emission recordings were resampled into common coordinates.
We used the program Display (http://www.bic.mni.mcgill.ca/software/distribution) to draw templates of regions of interest (ROIs) bilaterally on the average MR images, identifying the hippocampus and the insular, cingulate, and ventral medial prefrontal cortices. Because of central and cortical atrophy in the elderly subjects, each of the 8 regions was adjusted to the individual resampled MR images. The raphé nuclei were not visible on MR images and were outlined on the individual images in each subject.
Data Analyses
Hypotime Method.
In the case of negligible input from the circulation, the tissue time–activity curves of the radioligand are dominated by washout from the brain regions of uptake. The method specifically developed to take this condition into account, Hypotime, is based only on the differences among washout rates from regions with different properties of binding, blood flow rates, and blood–brain barrier permeability. To determine the rates of washout from regions of binding and a reference region of no specific binding, the following linked differential equations were solved, assuming homogeneity of single voxels. The first equation gives the rate of decay of tracer in a voxel of binding, Eq. 1where
Eq. 1where  is the total quantity of tracer radioligand in nonbinding (subscript 2) and binding (subscript 3) compartments of a voxel having specific binding (subsubscript 1) of the tracer radioligand (23). As the fractional clearance or washout rate from the radioligand compartments in a region of specific binding, the rate constant
is the total quantity of tracer radioligand in nonbinding (subscript 2) and binding (subscript 3) compartments of a voxel having specific binding (subsubscript 1) of the tracer radioligand (23). As the fractional clearance or washout rate from the radioligand compartments in a region of specific binding, the rate constant  for an apparent first-order decay is defined as,
for an apparent first-order decay is defined as, Eq. 2provided the rates of association and dissociation are sufficiently rapid, as assumed for the 1-compartment first-order kinetics where
Eq. 2provided the rates of association and dissociation are sufficiently rapid, as assumed for the 1-compartment first-order kinetics where  is the rate of removal across the blood–brain barrier, and BPND is the binding potential relative to nondisplaceable tracer, equal to the ratio
is the rate of removal across the blood–brain barrier, and BPND is the binding potential relative to nondisplaceable tracer, equal to the ratio  . The rate constants
. The rate constants  and
and  are the constants of association and dissociation, respectively. For a voxel with a single extravascular tracer accumulation compartment (subscript 2) in a region of no specific binding (subsubscript 2), Equation 1 reduces to,
are the constants of association and dissociation, respectively. For a voxel with a single extravascular tracer accumulation compartment (subscript 2) in a region of no specific binding (subsubscript 2), Equation 1 reduces to, Eq. 3where
Eq. 3where  is the fractional clearance or washout rate of the tracer from such a reference voxel of no binding, and
is the fractional clearance or washout rate of the tracer from such a reference voxel of no binding, and  is the quantity of tracer in such a reference voxel. The solutions to each of these differential equations predict the monoexponential washout of the radioligand from the respective voxels. When T is the time of the last frame of the positron emission tomograms, the ratio between the twice-integrated and the once-integrated solutions to Equations 1 and 3 is the Hypotime measure, which has a unit of time but a magnitude less than real time (hence the term Hypotime). The value depends on the magnitudes of the decay constants
is the quantity of tracer in such a reference voxel. The solutions to each of these differential equations predict the monoexponential washout of the radioligand from the respective voxels. When T is the time of the last frame of the positron emission tomograms, the ratio between the twice-integrated and the once-integrated solutions to Equations 1 and 3 is the Hypotime measure, which has a unit of time but a magnitude less than real time (hence the term Hypotime). The value depends on the magnitudes of the decay constants  and
and  as follows,
as follows, Eq. 4where Θ is the Hypotime measure. The maximum value that Θ″ can attain is T, consistent with an infinitely high washout rate, and the minimum is T/2, consistent with absent washout and permanent deposit of the radioactivity in the tissue, in which case the chemical microsphere is now stuck permanently in the tissue and the BPND is infinitely high. The magnitude of this measure in voxels of no binding defines the reference value (
Eq. 4where Θ is the Hypotime measure. The maximum value that Θ″ can attain is T, consistent with an infinitely high washout rate, and the minimum is T/2, consistent with absent washout and permanent deposit of the radioactivity in the tissue, in which case the chemical microsphere is now stuck permanently in the tissue and the BPND is infinitely high. The magnitude of this measure in voxels of no binding defines the reference value ( ″) as,
″) as, Eq. 5where the rate constant
Eq. 5where the rate constant  is the relaxation constant of the reference region. The rate constant
is the relaxation constant of the reference region. The rate constant  incorporates the combined effects of blood flow, the physical distribution volume of the tracer (equal to the partition coefficient in the absence of specific binding), and the permeability of the blood–brain barrier to the tracer. These properties are expressed in the unidirectional clearance of the tracer. Equations 4 and 5 are not easily solved for
incorporates the combined effects of blood flow, the physical distribution volume of the tracer (equal to the partition coefficient in the absence of specific binding), and the permeability of the blood–brain barrier to the tracer. These properties are expressed in the unidirectional clearance of the tracer. Equations 4 and 5 are not easily solved for  or
or  but a solution is provided by the growth equation of Gompertz (24),
but a solution is provided by the growth equation of Gompertz (24), Eq. 6which, when expressed with the notation used here, yields the solutions,
Eq. 6which, when expressed with the notation used here, yields the solutions, Eq. 7where α is a scaling factor characteristic of the duration of integration, which is eliminated by the use of ratios. The clearance from a voxel in a binding region relative to the clearance observed in the reference region defines the clearance ratio
Eq. 7where α is a scaling factor characteristic of the duration of integration, which is eliminated by the use of ratios. The clearance from a voxel in a binding region relative to the clearance observed in the reference region defines the clearance ratio  , equal to the ratio
, equal to the ratio  , where
, where  is the clearance from a nonbinding voxel in the reference region,
is the clearance from a nonbinding voxel in the reference region, Eq. 8where R1 is the ratio between the voxel values at the time of the peak accumulation of radioactivity, relative to the reference region, when other terms have the meaning described above. The decay of the time–activity curves is thus given by the solution to Equations 2 and 8,
Eq. 8where R1 is the ratio between the voxel values at the time of the peak accumulation of radioactivity, relative to the reference region, when other terms have the meaning described above. The decay of the time–activity curves is thus given by the solution to Equations 2 and 8, Eq. 9where the symbols have the same meaning as above. The solution to Equation 9 is,
Eq. 9where the symbols have the same meaning as above. The solution to Equation 9 is, Eq. 10which yields the operational equation of the BPND when the expressions given in Equation 7 are inserted into Equation 10,
Eq. 10which yields the operational equation of the BPND when the expressions given in Equation 7 are inserted into Equation 10, Eq. 11which has a simpler solution when
Eq. 11which has a simpler solution when 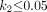 ,
, Eq. 12
Eq. 12
MLSRTM
The MLSRTM expressed in Equation 9 in the study by Zhou et al. (19) estimates the BPND by conventional weighted multilinear regression of operational equations of integral form to the regional records identified above. In the application of the method, we used the authors' Equation 9 without the spatial constraint added in that article to smooth regional values to a regional average.
Common Reference Region Mask
Washout from an ROI can be slow because the flow rate is low or because the distribution volume is high, or both. From the reference tissue we require a relationship between the rate of washout and the initial clearance (as a measure of blood flow) in a tissue that has the least binding possible. Thus 2 requirements must be fulfilled of the reference, that is, that the tissue has no specific binding and that the composition of the tissue is homogeneous to provide the most precise relationship between the intial tracer clearance to the reference tissue and the Hypotime variable in the reference tissue.
The calculated Θ″ values of all voxels were used to segment the tissue into groups on the basis of major subdivisions of values and hence were used to specify the region of presumed absence of specific binding. The Θ″ map confirmed the suspected heterogeneity of cerebellar uptake, with the lowest Θ″ values in cortex and vermis and the highest washout rates in white matter. The mask was delineated to match the white matter of the cerebellum.
RESULTS
Reference Region Mask
Voxel maps of the Hypotime measure Θ″, calculated according to Equation 4, identified a region of no specific binding of the radioligand in the cerebellum. The absence of specific binding was defined as the presence of the highest values of the Hypotime measure associated with the white matter regions of cerebellum. In the distribution of Θ″ values, we chose the region corresponding to the highest 8% of the values as the reference mask with the least regional heterogeneity and the highest blood flow because of the least specific binding. On visual inspection, the voxels included in the mask remained within the anatomic boundaries of the white matter of the cerebellum. Figure 1 presents the Θ″ map and the cerebellar mask.
Θ″ map (left) and cerebellar mask (right).
11C-WAY Uptake and Clearance from Regions
Figure 2 illustrates the average time–activity curves of the groups of young and elderly subjects in the hippocampus, insula, and cingulate gyrus bilaterally. In these regionally averaged records, the curves show that the latter part of the washout curves declines monoexponentially. In general, the level of radioactivity in the selected regions was higher in the group of young subjects than in the elderly group.
Time–activity curves from hippocampus, insula, and cingulate in young (A) and elderly (B) subjects. Abscissa, time (min); ordinate, log activity as fraction of average area under the curves of activity in cerebellum. AUC = area under curve.
Figure 3 shows the distribution of R1 ratios based on frames 5–9 in 2,000 randomly selected voxels in each subject in the groups of young and elderly volunteers. The ratios R1 were obtained from the peak values of the 11C-WAY uptake images (plotted in the abscissa) versus ratios obtained from the images of the water distribution (plotted on the ordinate), as calculated according to Equation 7. The figure demonstrates the approximately linear relationship between the rapid and brief initial clearances of the 2 tracers from the circulation to the tissue. The relationship confirms the flow-limitation of the initial deposit of 11C-WAY presumed in the present application.
Accumulated radioactivity of water as index of cerebral blood flow, relative to cerebellum, vs. peak accumulation of 11C-WAY, relative to cerebellum, in randomly selected voxels in groups of young (A) and elderly (B) subjects. Abscissae are R1 ratios determined from 11C-WAY; ordinates are R1 ratios determined from water.
BPND by Hypotime Method and MLSRTM
Figure 4 shows the average parametric maps of the BPND determined in the 7 young and 12 elderly volunteers by the Hypotime method and MLSRTM. The distribution of receptor binding showed the highest values in the hippocampus and insula, with values on the order of 3–4 obtained by the Hypotime method and on the order of 2–3 obtained by the MLSRTM. In the raphé nuclei, BPND averaged 2 and 1, respectively. The average values of the BPND in basal ganglia and cerebellum were negligible by both methods. BPND maps obtained by the Hypotime method demonstrated the clearest discrimination among regions. The average magnitudes of BPND, determined by parametric Hypotime and MLSRTM analyses in the 9 ROIs in the 2 groups, are listed in Table 1.
Parametric maps of BPND of radioligand 11C-WAY in selected sections of brain tissue determined by 2 different methods, Hypotime and MLSRTM. Hypotime maps are shown to left, with columns with young and elderly subjects. MLSRTM maps are shown to right, with columns with young and elderly subjects.
Average Magnitudes of BPND, Determined by Parametric Hypotime and MLSRTM Analyses
The same average magnitudes of BPND are shown in Figure 5 as histograms of the distribution of BPND in the young and elderly for the Hypotime method and MLSRTM. By the Hypotime method, the regional BPND values in the medial temporal regions were greater in the young than in the elderly subjects, but age-related differences in other regions were smaller. The MLSRTM did not reveal differences of BPND between the 2 age groups. The linear correlation between the distributions of cortical BPND values by the 2 methods is shown in Figure 6.
Histograms of distribution of BPND in selected ROIs in groups of young and elderly adults measured by Hypotime method (A) and MLSRTM (B).
Scatterplot of BPND values of 9 ROIs obtained by Hypotime method and MLSRTM based on 12 elderly and 7 young healthy subjects.
DISCUSSION
The serotonin 5HT1A receptor is of interest in studies of development, plasticity, and memory processes in the human brain (25–27), but the quantification of these receptors has been fraught with difficulty from the first publication of maps of BPND with the radioligand 11C-WAY. In the present article, we argue that an appropriate method of analysis of 11C-WAY binding must take the rapid disappearance of the radioligand 11C-WAY from the circulation into account. We tested the results of the Hypotime method that uses the specific washout rates from different brain regions to estimate the magnitude of the volumes of distribution that delay the washout. The computationally efficient and accurate method involves no regression, and we found that reliable BPND can be obtained. The distribution of receptor BPND obtained with Hypotime in different brain regions is clearly consistent with previous results of autoradiography published by Hall et al. (17) and summarized for selected regions in Figure 7.
Histogram of averaged autoradiographic locations of 5HT1A receptors in postmortem human brain obtained with 3H-WAY-100635 (17).
The main feature of the method is the calculation of BPND from washout rates corrected for different blood flow rates on the basis of initial deposit of tracer. The initial deposit is flow-limited as shown in Figure 3, which shows that more than 60% of all initial deposit ratios (R1) reside on a line of identity between the ratios of the radioligand and water as a flow tracer. Both the basic Hypotime measure (Θ″) and resulting BPND are model-independent as determined directly by calculation rather than by regression to a model-derived formula. This means that the BPND values obtained by parametric and ROI-based analyses do not diverge significantly as they tend to do when regression is involved.
The question is whether the results differ significantly from those of other methods, and also whether the variability of the BPND assessed in different subjects by different researchers changes significantly when the Hypotime method is used in place of more conventional methods. A potential concern in receptor mapping is the test–retest variability of the results. The differences in BPND values between age groups obtained by different kinetic models should be weighed against the reproducibility in previous receptor studies with 11C-WAY. We found the variability to be in the range of 2%−15%, excluding the raphè (8,11), which is consistent with test–retest values in receptor studies of 11C-labeled 3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile, 18F-2′-methoxyphenyl-(N-2′-pyridinyl)-p-18F-fluoro-benzamidoethylpiperazine, and 18F-altanserin.
We compared the results of the Hypotime method with the results of other methods using a reference region. In a previous study, we compared the results of the MLSRTM with other methods used in current research (9), and in this study we compared Hypotime with the MLSRTM. With Hypotime, the highest values were found in the hippocampus and insula, whereas with the MLSRTM, no significant differences were observed among the regional values. The BPND maps obtained by Hypotime are consistent with the findings by autoradiography of receptor density in slices of brain obtained postmortem that show regionally differentiated binding, but this distribution is not matched by the in vivo receptor map prepared with the MLSRTM from the same PET images of the radioligand uptake.
The Hypotime method demonstrated only subtle differences in the BPND from 30 to 55 min of the scan time as shown in Figure 8 of time-stability analysis based on washout in the group of young subjects, which indicates that a 60-min scan time is sufficient for the quantification of BPND using the radioligand 11C-WAY.
Time-stability analysis based on washout in group of young subjects.
The Hypotime method was useful also for the delineation of a proper reference region devoid of 5HT1A receptors. It is possible that several kinds of ROIs could be defined by means of the functional Θ″ values rather than by comparatively crude anatomic boundaries, especially in structures with a heterogeneously distributed density of receptors in the layers of cortices such as the hippocampus.
The Hypotime method demonstrated a correlation with age in the regions of the highest BPND, such as the hippocampus and insula. In general, however, an age effect must be regarded with caution because of the multiple interpretations of BPND and the unknown relative contributions from changes in affinity, concentration of endogenous ligand, and receptor density.
CONCLUSION
For tracers such as 11C-WAY used here, conventional reference region methods are subject to the variability associated with early disappearance of the radioligand from the circulation and the associated uncertain determination of late concentrations in the circulation. In contrast, the Hypotime method gives regionally specific values of binding to the serotonin 5HT1A receptors, in the form of receptor availabilities. The method is simple and computationally convenient, because no regression is involved. The effect of age is seen most significantly only in the regions of the highest receptor availabilities.
Acknowledgments
We thank the University of Aarhus, Denmark's National Science Foundation, and the Medical Research Council of Denmark for support of this study. This work was supported in part by a fellowship from the Clinical Institute of the University of Aarhus, a Center of Excellence grant from Denmark's National Science Foundation to CFIN, and operating grants from the Medical Research Council of Denmark.
Footnotes
-
COPYRIGHT © 2009 by the Society of Nuclear Medicine, Inc.
References
- Received for publication April 9, 2008.
- Accepted for publication April 9, 2009.















